Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
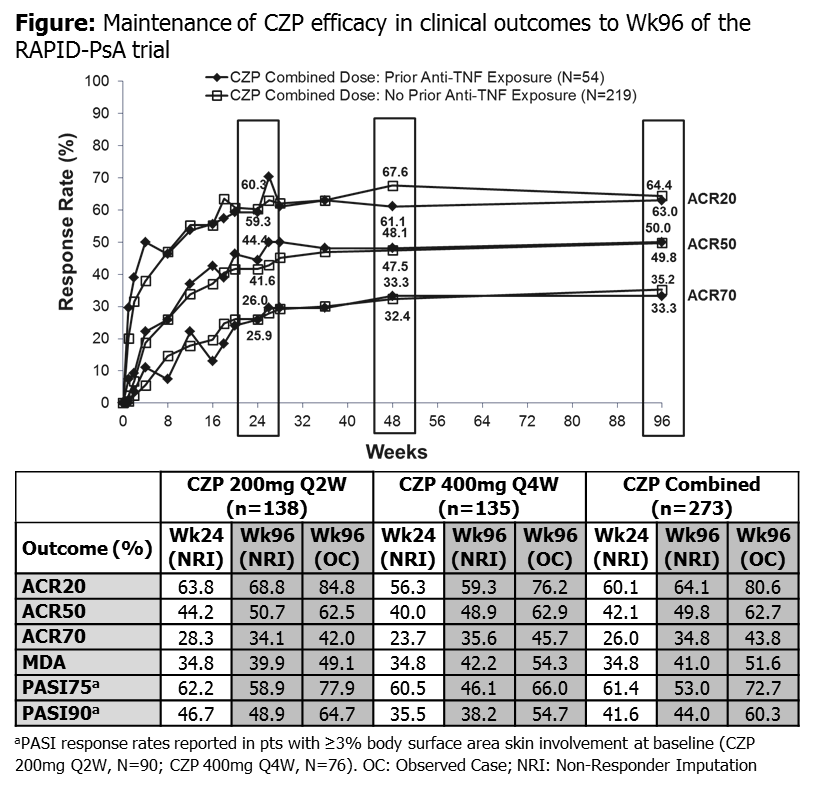
Previous reports of RAPID-PsA (NCT01087788) demonstrated efficacy and safety of certolizumab pegol (CZP) over 48 weeks (wks) in psoriatic arthritis (PsA) patients (pts), including pts with prior anti-TNF therapy.1 Here, we report the efficacy and safety of CZP in PsA from a 96-wk interim data cut of RAPID-PsA.
Methods
RAPID-PsA1 is double-blind and placebo (PBO)-controlled to Wk24, dose-blind to Wk48 and open-label (OL) to Wk216. Pts had active PsA and had failed ≥1 DMARD. Pts originally randomized to CZP (200mg Q2W/400mg Q4W, following 400mg loading dose [LD] at Wks 0, 2, 4) continued on assigned dose in the OL phase; PBO pts entering dose-blind phase were re-randomized to CZP LD followed by CZP 200mg Q2W or 400mg Q4W after Wk24 or, for non-responders, Wk16. We present efficacy data for pts originally randomized to CZP (combined dose regimens). Primary clinical endpoint was Wk12 ACR20 response.1 Other endpoints included ACR and PASI responses, HAQ-DI, pain and minimal disease activity (MDA)2 at Wk96, and ACR responses in pts with/without prior anti-TNF exposure. Data are shown as observed case and with imputation (NRI for categorical measures; LOCF for continuous measures). Safety set consists of pts treated with ≥1 dose of CZP to Wk96.
Results
409 pts were randomized and 273 received CZP from Wk0. 54 (19.8%) CZP pts had prior anti-TNF exposure with similar baseline (BL) characteristics to pts without. Of CZP-randomized pts, 91% completed Wk24, 87% Wk48 and 80% Wk96. ACR and MDA responses were maintained in both dosing regimens from Wk24 to Wk96 (Figure). ACR responses were similar in pts with/without prior anti-TNF exposure (Figure). Pts randomized to PBO who switched to CZP displayed rapid clinical improvements that were maintained to Wk96. In pts with ≥3% skin involvement at BL (N=166, 60.8% CZP pts) PASI responses were maintained to Wk96 (Table). Improvements in PROs were maintained to Wk96 (Change from BL at Wk24 and Wk96: HAQ-DI: -0.48 vs -0.52; pain: -28.5 vs -31.3). In the safety set (N=393) total CZP exposure was 606 pt-yrs. Adverse events (AEs) occurred in 345 pts (87.8%; event rate [ER] per 100 pt-yrs=329.8) and serious AEs (SAEs) in 67 (17.0%; ER=14.5). SAEs included malignancies in 4 pts (1.0%; ER=0.7) (2 cases of breast cancer, 1 lymphoma, 1 stage 0 in situ cervix carcinoma) and serious infections in 16 pts (4.1%; ER=3.3). 6 deaths were reported in the overall 96-wk period (1.5%) (2 cardiac disorders, 1 sudden death, 1 infection, 1 breast cancer, 1 lymphoma).
Conclusion
Clinical efficacy of CZP was maintained through both the dose-blind and OL phases of RAPID-PsA. Efficacy was maintained in both dosing regimens and in both TNF-naïve and TNF-experienced pts. The safety profile was in line with previous reports from RAPID-PsA, with no new safety signals observed with increased exposure.
References:
1. Mease P.J. Arthritis Rheum 2013;65(10):S132-S133
2. Coates L. Ann Rheum 2010;69(1):48-53
Disclosure:
P. J. Mease,
Abbott, AbbVie, Amgen, BiogenIdec, BMS, Celgene, Crescendo, Genentech, Janssen, Lilly, Merck, Novartis, Pfizer, UCB Pharma, Vertex,
2,
Abbott, AbbVie, Amgen, BiogenIdec, BMS, Celgene, Covagen, Crescendo, Genentech, Janssen, Lilly, Merck, Novartis, Pfizer, UCB Pharma, Vertex,
5,
Abbott, AbbVie, Amgen, BiogenIdec, BMS, Crescendo, Genentech, Janssen, Lilly, Pfizer, UCB Pharma,
8;
R. Fleischmann,
Genetech Inc, Roche, Abbott, Amgen, UCB Pharma, Pfizer, BMS, Lilly, Sanofi-Aventis, MSD, Novartis, BiogenIdec, Astellas, AstraZeneca, Janssen,
2,
Roche, Abbott, Amgen, UCB Pharma, Pfizer, BMS, Lilly, Sanofi-Aventis, Novartis, Astellas, AstraZeneca, Janssen,
5;
J. Wollenhaupt,
UCB Pharma,
5,
UCB Pharm,
2;
A. A. Deodhar,
Abbott, Amgen, Janssen, Novartis, Pfizer and UCB Pharma,
2,
Abbott, Amgen, Janssen, Novartis, Pfizer and UCB Pharma,
5;
D. D. Gladman,
Abbott, Bristol Myers Squibb, Celgene, Johnson & Johnson, MSD, Novartis, Pfizer, and UCB Pharma,
2,
Abbott, Bristol Myers Squibb, Celgene, Johnson & Johnson, MSD, Novartis, Pfizer, and UCB Pharma,
5;
B. Hoepken,
UCB Pharma,
3;
L. Peterson,
UCB Pharma,
3;
D. M. van der Heijde,
AbbVie, Amgen, AstraZeneca, Augurex, BMS, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, Galapagos, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Vertex,
5,
Imaging Rheumatology bv,
9.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-certolizumab-pegol-over-96-weeks-in-patients-with-psoriatic-arthritis-with-and-without-prior-tumor-necrosis-factor-inhibitor-exposure/