Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Certolizumab pegol (CZP) has demonstrated clinical efficacy in patients with active non-radiographic axial spondyloarthritis (nr-axSpA) and objective signs of inflammation (sacroiliitis on MRI and/or elevated C-reactive protein levels) treated during the 52-week placebo-controlled period of C axSpAnd.1 We evaluate long-term safety and changes in clinical outcomes in patients with active nr‑axSpA treated with CZP for up to 3 years during the C-axSpAnd double-blind period and open-label (OL) safety follow-up extension (SFE).
Methods: C-axSpAnd (NCT02552212) was a 3-year, phase 3, multicenter study consisting of a 1-year double-blind, placebo-controlled period (Weeks 0–52) followed by a 2-year OL SFE (Weeks 52–156).1 Patients were randomized 1:1 to placebo (PBO) or CZP (400 mg at Weeks 0, 2, 4, then 200 mg every 2 weeks [Q2W]), which they received in addition to non-biologic background medication for 52 weeks. Switching to OL CZP (or other biologics) was permitted at any point. At Week 52, patients completing the double-blind phase (on CZP, PBO or OL CZP) who enrolled into the SFE received OL CZP 200 mg Q2W for an additional 104 weeks. We report safety and clinical outcomes (Ankylosing Spondylitis Disease Activity Score [ASDAS], BASDAI and BASFI, ASDAS Major Improvement [ASDAS-MI], Assessment of SpondyloArthritis international Society 40% response [ASAS40]) up to Week 156 for patients who entered the SFE according to whether they were initially randomized to CZP or PBO. As efficacy outcomes for the SFE were assessed at Weeks 52 and 156 only, data are reported as observed case (OC).
Results: 243/317 patients who entered C-axSpAnd continued into the SFE, including 120 patients from the CZP-randomized arm and 123 from the PBO-randomized arm. During the double-blind period, prior to SFE entry, 75/123 PBO patients had switched to OL CZP, compared to 10/120 of CZP patients. In total, 102/120 (85.0%) and 104/123 (84.6%) SFE patients from the CZP- and PBO-randomized arms, respectively, completed the study up to Week 156.
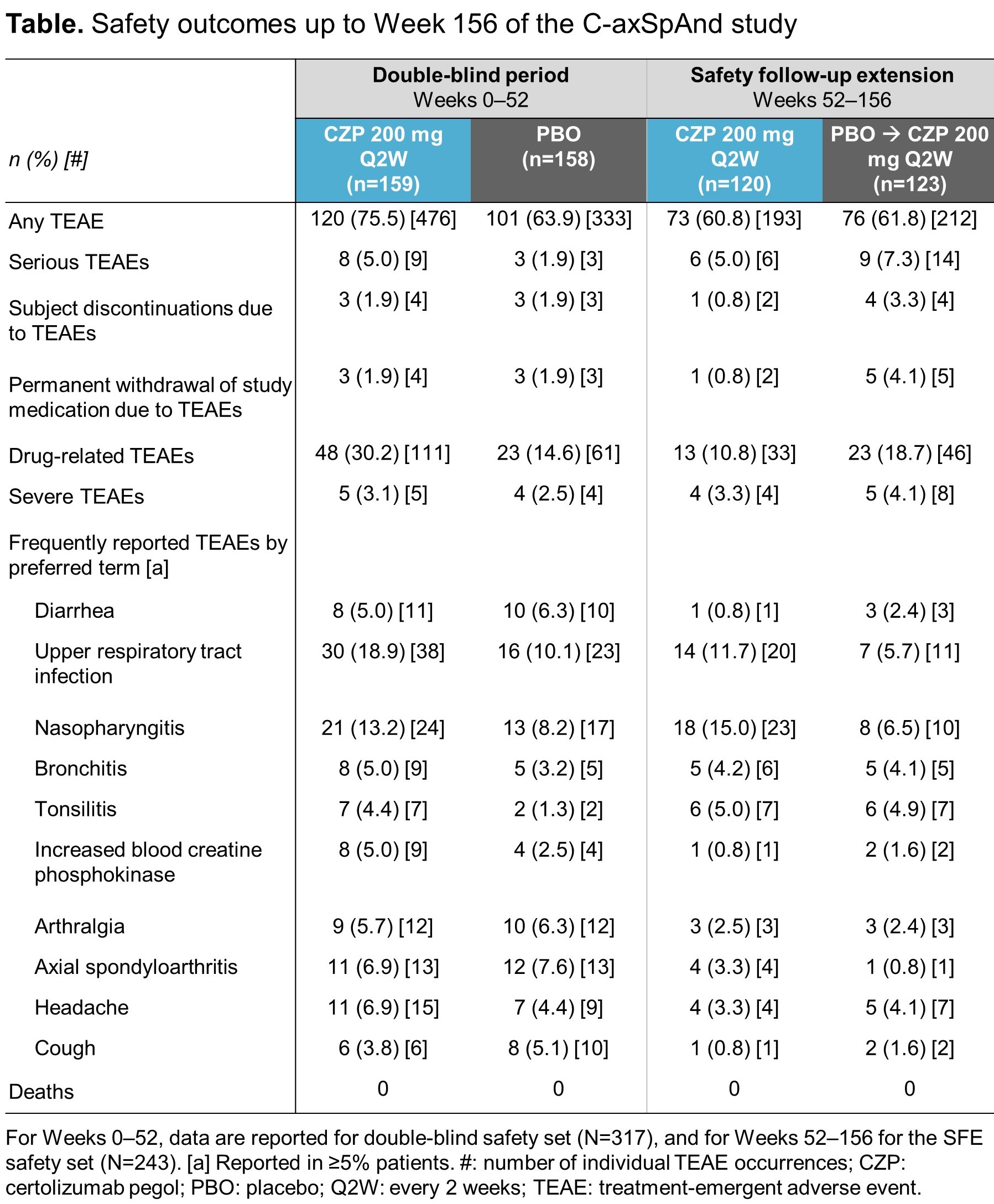
During the SFE, 405 TEAEs were reported for 149 (61.3%) patients, including 20 serious TEAEs in 15 (6.2%) patients (Table). Over a period of up to 3 years of CZP treatment, no new safety signal was identified.
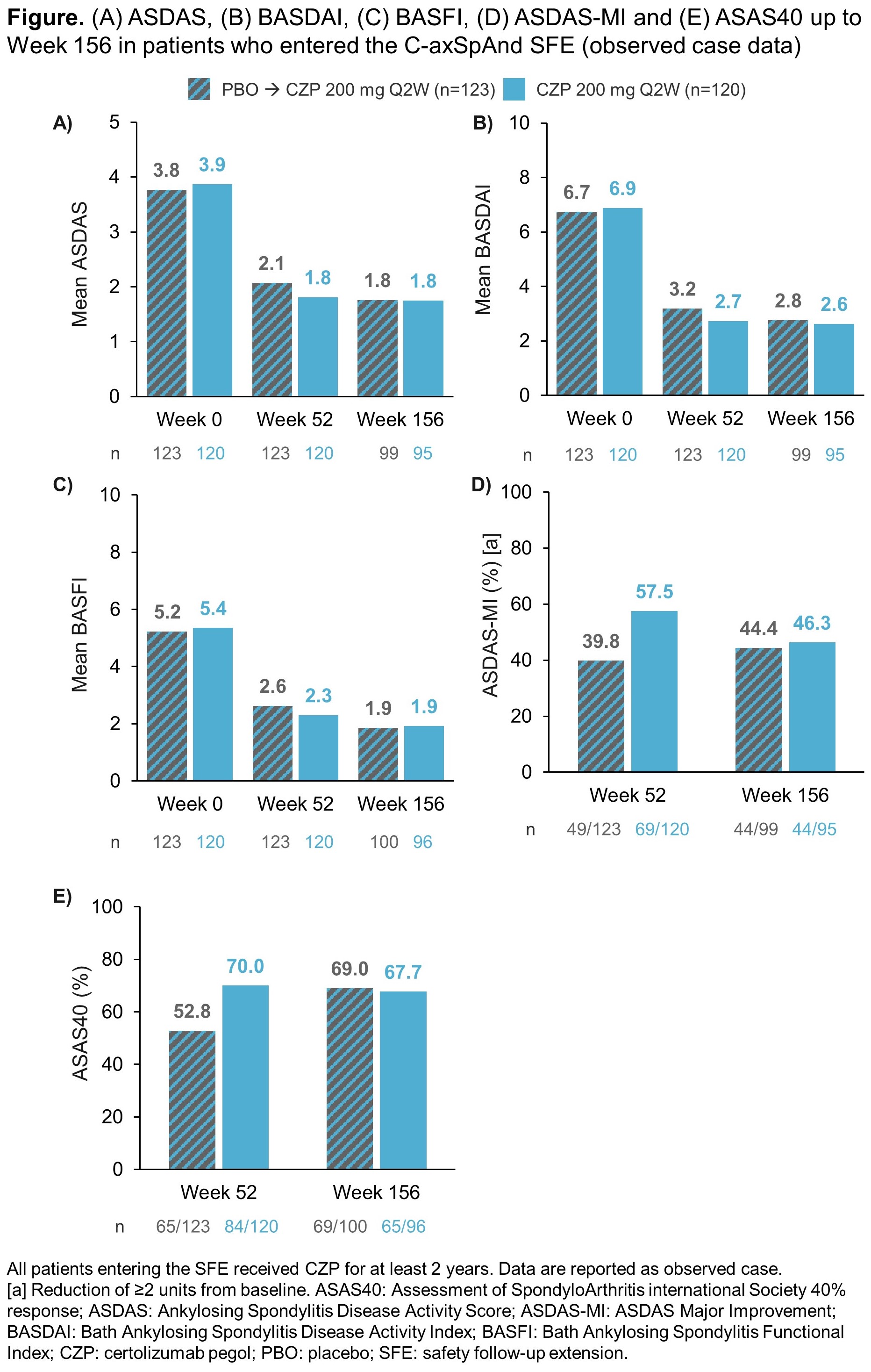
At Week 52 (SFE baseline), using OC data, there were substantial improvements from Week 0 in ASDAS, BASDAI and BASFI, and in ASDAS-MI and ASAS40 response rates (Figure). As the majority of PBO-randomized patients had switched to OL CZP during the double-blind period, improvements were also observed in this arm at Week 52. Responses were maintained to Week 156 in both arms, and by Week 156, improvements across all reported efficacy outcomes in patients initially randomized to PBO were comparable to those initially randomized to CZP (Figure).
Conclusion: This analysis reports safety and clinical outcomes in the nr-axSpA patients who entered the C-axSpAnd SFE and received OL CZP treatment. During the SFE, CZP was shown to be well tolerated in patients with nr-axSpA, in line with 1-year data.1 In patients receiving CZP treatment for up to 3 years, observed improvements in signs and symptoms after 1 year were maintained up to 3 years.
References: 1. Deodhar A. Arthritis Rheum 2019;71:1101–11.
To cite this abstract in AMA style:
van der Heijde D, Gensler L, Maksymowych W, Landewé R, Rudwaleit M, Bauer L, Hoepken B, Kumke T, Kim M, Deodhar A. Long-Term Safety and Efficacy of Certolizumab Pegol in Patients with Active Non‑Radiographic Axial Spondyloarthritis: 3-Year Results from a Phase 3 Multicenter Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-certolizumab-pegol-in-patients-with-active-non%e2%80%91radiographic-axial-spondyloarthritis-3-year-results-from-a-phase-3-multicenter-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-certolizumab-pegol-in-patients-with-active-non%e2%80%91radiographic-axial-spondyloarthritis-3-year-results-from-a-phase-3-multicenter-study/