Session Information
Date: Monday, November 14, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster III: Outcomes
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Many clinicians use belimumab as a maintenance therapy of SLE, but there is scarce data on belimumab drug retention rate and safety/effectiveness profile in real world practice.
Therefore, we conducted this single center retrospective cohort to analyze belimumab safety profile in our hospital.
Methods: We retrospectively analyzed patient with SLE who was treated with belimumab at our institute.
We included patient with SLE who were followed up at our institute between January 2006 and March 2022.
We analyzed the drug retention rate, LDAS achievement ratio, flare free ratio and severe infection ratio using Kaplan–Meier method and log rank test. In addition, we analyzed risk and protective factors for early LLDAS achievement (LLDAS achievement < 250 days after belimumab initiation) using cox proportional hazard model.
Results: Of the 567 patients with SLE followed up in our hospital, 95 used belimumab as a maintenance therapy and total of 92 patients with SLE were included in the study.
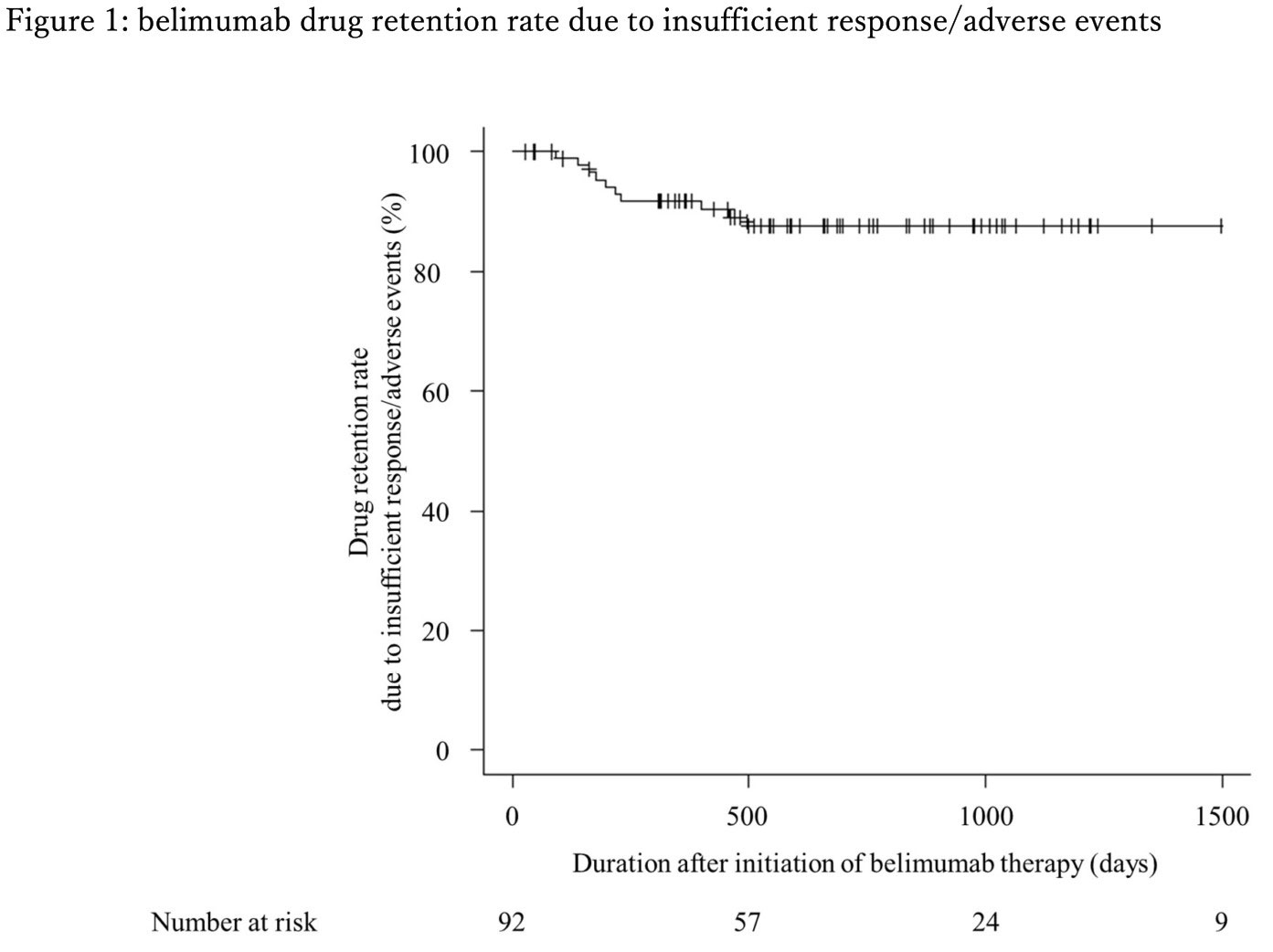
Drug retention rate of belimumab was around 90% at 52 weeks and over 80% at day 1000 after initiation of belimumab.
Severe infection occurred in less than 10% at week 52 and around 20% of the patient at day 1000 after initiation of belimumab.
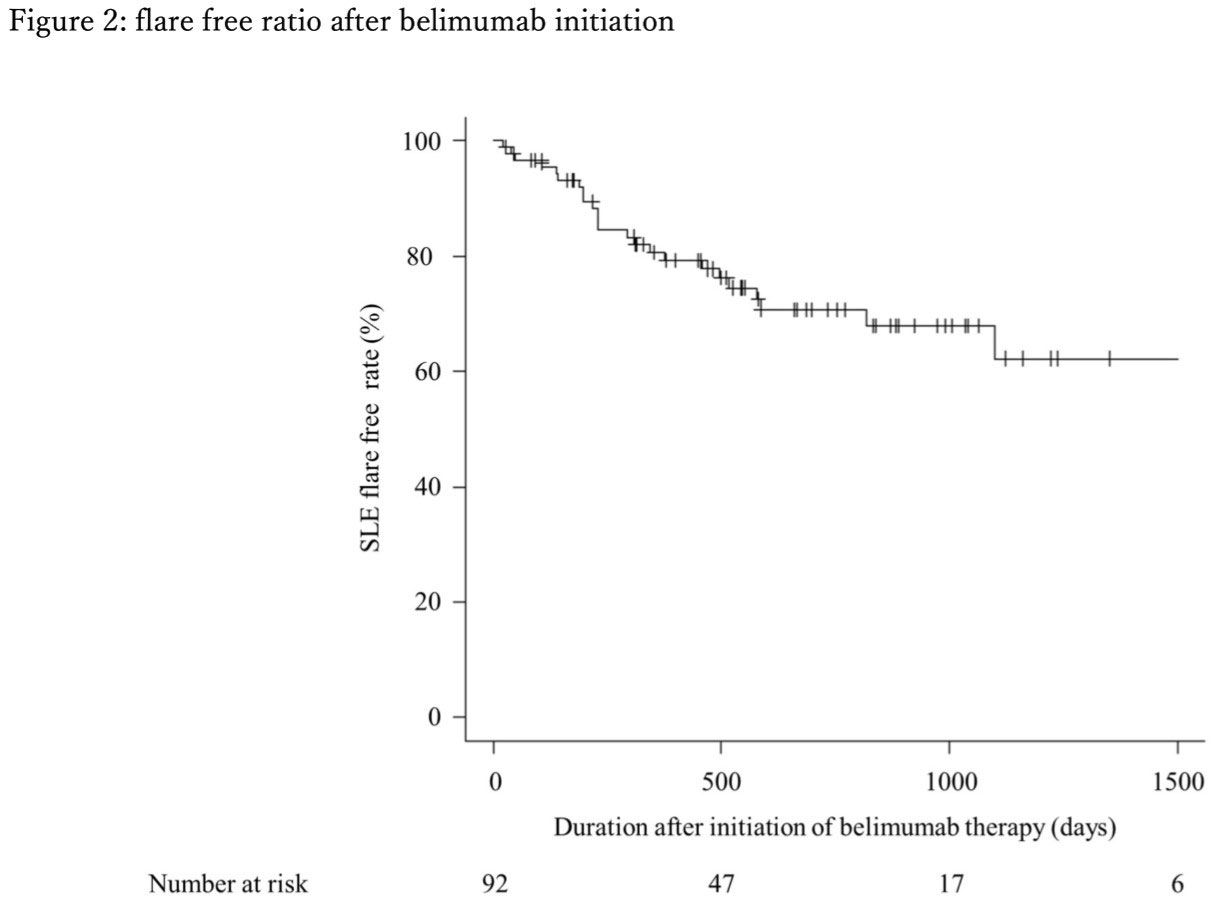
Even though 25.8% of the patient with SLE flared at least once during the follow up period, more than 50% of the patient achieved LLDAS at 52 weeks and more than 80% achieved LLDAS at 1000 days after initiation of belimumab therapy.
Presence of renal manifestation, prior treatment with mPSL pulse therapy and prior treatment with PSL 1mg/kg/day affected negatively and HCQ use on the day of belimumab initiation affected positively to early LLDAS achievement. (renal manifestation: HR 0.35 95% CI 0.12-1.03, p=0.06, prior treatment with mPSL pulse therapy: HR 0.17, 95% CI 0.05-0.62, p< 0,01, prior treatment with PSL >1mg/kg/day: HR 0.15 95% CI 0.03-0.7, p=0.03, HCQ use on the day of belimumab therapy: HR 4.28 95% CI 1.37-13.36, p=0.01)
Conclusion: Belimumab has high drug retention rate. Even though some experience lupus flare in short term, most of the patient on belimumab therapy achieved LLDAS during the follow up. Therefore, continuation of belimumab is recommended if it is not contraindicated.
To cite this abstract in AMA style:
Nakai T, Fukui S, Asano T, Iwata F, Ozawa H, Kawaai S, Ikeda Y, Tamaki H, Kishimoto M, YAMAGUCHI K, Okada M. Long Term Safety and Effectiveness of Belimumab Therapy in Patient with SLE: A Single Center Retrospective Analysis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-effectiveness-of-belimumab-therapy-in-patient-with-sle-a-single-center-retrospective-analysis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-effectiveness-of-belimumab-therapy-in-patient-with-sle-a-single-center-retrospective-analysis/