Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: In rheumatoid arthritis (RA), anti-TNF therapy is considered following 3-6 months of failed methotrexate (MTX) treatment. Some patients (pts), particularly those with many risk factors, may benefit from earlier intervention with anti-TNF+MTX, as this combination maximizes the likelihood of response. This analysis assessed, on a group level, whether there is a long-term advantage for early RA pts treated with initial and continued adalimumab (ADA)+MTX vs those treated with initial placebo (PBO)+MTX and who either continued unaltered therapy or rapidly added ADA following an inadequate response (IR).
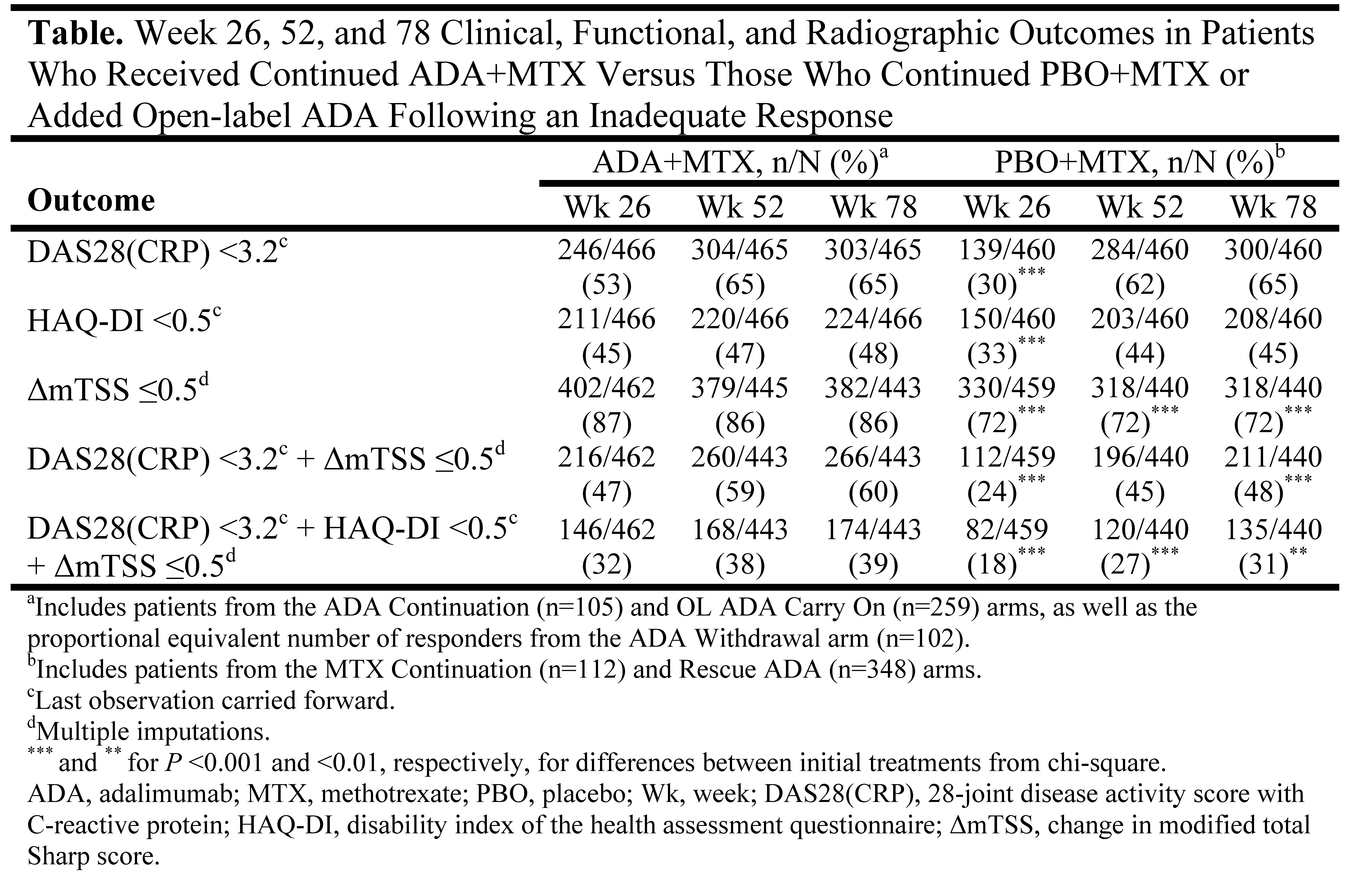
Methods: OPTIMA was a 78-week (wk), phase 4, randomized, controlled trial of initial ADA+MTX vs PBO+MTX in MTX-naïve pts ≥18 years, <1 year disease duration, and active RA. Therapy adjustments were made at wk 26 on the basis of achieving a target of stable low disease activity [LDA, defined for this study as DAS28(CRP) <3.2] at wks 22 and 26 (Period 1, P1): ADA+MTX-responders (R) were re-randomized to either withdraw or continue ADA (ADA Withdrawal and Continuation arms, respectively) and PBO+MTX-R continued randomized therapy (MTX Continuation arm) for an additional 52 wks (P2); IR-pts received open-label (OL) ADA+MTX during P2 (OL ADA Carry On and Rescue ADA arms). This post hoc analysis evaluated the proportion of pts who entered P2 and were in LDA, had normal function (HAQ-DI <0.5), and/or the absence of radiographic progression (ΔmTSS ≤0.5) at wk 78 by initial treatment group (ie, the ADA+MTX group includes ADA Withdrawal, Continuation, and OL Carry On arms; the PBO+MTX group includes MTX Continuation and Rescue ADA arms). To account for the full population of P1 ADA+MTX-R, those who withdrew ADA during P2 were replaced by an equivalent proportion of R from the ADA Continuation arm.
Results: Among pts who entered P2 (ADA+MTX, N=466; PBO+MTX, N=460), significantly more were in LDA, had normal function, and/or the absence of radiographic progression following 26 wks of treatment with ADA+MTX vs PBO+MTX (Table). Differences in clinical and functional outcomes between initial treatment groups disappeared following an additional 26 or 52 wks of treatment, during which time PBO+MTX-IR (n=348) were switched to OL ADA+MTX. Although the addition of OL ADA to PBO+MTX-IR slowed radiographic progression during P2, more pts who received ADA+MTX from baseline had no radiographic progression at wk 78 than pts who received initial PBO+MTX.
Conclusion: Early RA pts treated with PBO+MTX were able to achieve comparable long-term clinical and functional outcomes on a group level to those who began treatment with the combination of ADA+MTX, but only if therapy was optimized by the addition of ADA in PBO+MTX-IR. Still, continued ADA+MTX therapy conferred a radiographic benefit over 78 wks, although the difference did not appear to translate to an additional functional benefit.
Disclosure:
R. Fleischmann,
Abbott, Pfizer, Merck, Roche, UCB, Celgene, Centocor-Jannsen, Amgen, AstraZeneca, BMS, Lilly, and Novartis. ,
2,
Abbott, Pfizer, Merck, Roche, UCB, Celgene, Centocor-Jannsen, Amgen, AstraZeneca, BMS, Lilly, and Novartis. ,
5;
R. F. van Vollenhoven,
Abbott, BMS, Glaxo SmithKline, Human Genome Sciences, Merck, Pfizer, Roche, and UCB Pharma,
5,
Abbott, BMS, Glaxo SmithKline, Human Genome Sciences, Merck, Pfizer, Roche, and UCB Pharma,
2;
J. S. Smolen,
Abbott, Amgen, Astra-Zeneca, BMS, Celgene Centocor-Janssen, Glaxo, Lilly, Pfizer (Wyeth), MSD (Schering-Plough), Novo-Nordisk, Roche, Sandoz, and UCB,
2,
Abbott, Amgen, Astra-Zeneca, BMS, Celgene Centocor-Janssen, Glaxo, Lilly, Pfizer (Wyeth), MSD (Schering-Plough), Novo-Nordisk, Roche, Sandoz, and UCB,
5;
P. Emery,
Abbott, Merck, Pfizer, UCB, Roche, and BMS,
5;
S. Florentinus,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
S. S. Rathmann,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
H. Kupper,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
A. Kavanaugh,
Abbott, Amgen, Astra-Zeneca, BMS, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB,
5,
Abbott, Amgen, Astra-Zeneca, BMS, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB,
2.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-outcomes-of-early-rheumatoid-arthritis-patients-initiated-with-adalimumab-plus-methotrexate-compared-with-methotrexate-alone-following-a-targeted-treatment-approach/