Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Improving and preserving patient (pt) physical function is an important goal in managing psoriatic arthritis (PsA). Apremilast (APR) has been shown to improve signs and symptoms, quality of life, and functional disability in DMARD/biologic-experienced (PALACE 1-3 [PAL1-3]) and DMARD-naive (PALACE 4 [PAL4]) pts with active PsA. We report long-term functional status for pts continuing APR up to 3 yrs.Methods: Pts were randomized (1:1:1) to placebo (PBO), APR 30 mg BID (APR30), or 20 mg BID (APR20). PBO pts were re-randomized to APR30 or APR20 at Wk 16 (early escape) or Wk 24. HAQ-DI scores were assessed throughout the study for mean change from BL, proportion of pts reaching MCID, and proportion reaching scores ≤1.0 (below clinically significant disability), ≤0.5 (minimal disability), and ≤0.25 (general population). PAL1-3 data were pooled. Wk 16 APR30 data vs PBO were analyzed by LOCF. Wk 156 data are as observed. Mean change and MCID outcomes are for all pts receiving APR30 at any time during the study; disability level data are for pts randomized to APR30 at BL.
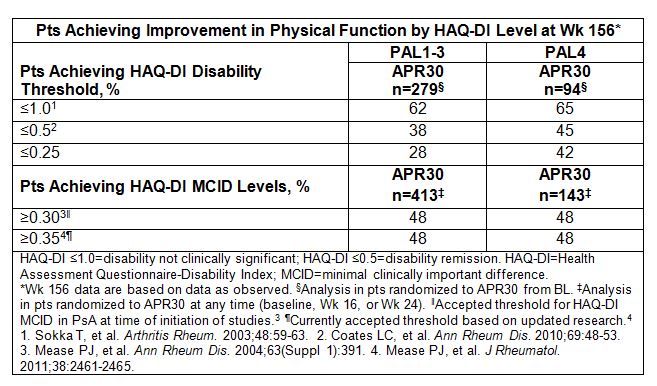
Results: PAL1-3 (biologic/DMARD experienced) and PAL4 (DMARD naïve) pts were similar in BL SJC/TJC and DAS-28 (CRP), indicating active PsA. PAL1-3 pts had longer mean duration of PsA and psoriasis, higher PASI scores, and greater corticosteroid use at BL. Despite longer PsA duration in PAL1-3 vs PAL4 (7.4 vs 3.4 yrs), clinically significant BL physical disability was similar: mean HAQ-DI 1.2 (PAL1-3) vs 1.1 (PAL4). At Wk 16, physical function significantly improved with APR30 vs PBO: mean HAQ-DI change −0.23 vs −0.08 (PAL1-3) and −0.21 vs 0.03 (PAL4) (both P<0.0001). Significantly more APR30 vs PBO pts reached MCID ≥0.30 and ≥0.35 in both populations. Marked disability at BL was seen in some pts randomized to APR30, with HAQ-DI scores up to 2.63-2.88. More PAL1-3 vs PAL4 APR30 pts had BL HAQ-DI >1.0 (60% vs 54%), >1.5 (31% vs 21%; marked difficulty/need for assistive devices), and >1.75 (19% vs 10%; major disability), highlighting need for early, effective treatment. Few APR30 pts in PAL1-3 or PAL4 had BL scores ≤0.5 (18%-22%) or ≤0.25 (10%-14%). At Wk 16, disability levels shifted; more APR30 vs PBO pts achieved HAQ-DI ≤1.0 (56% vs 48% [PAL1-3]; 60% vs 52% [PAL4]). At Wk 156 in PAL1-3 and PAL4 pts, 62% and 65% achieved HAQ-DI ≤1.0, 38% and 45% achieved ≤0.5, and 28% and 42% reached ≤0.25 (Table). LOCF analyses confirmed Wk 156 results.
Conclusion: With APR30 treatment, physical disability improved early and functionality was maintained over time up to 3 yrs. Most pts achieved HAQ-DI ≤1.0; many attained minimal/mild physical impairment. Over 40% of DMARD-naïve pts randomized to APR30 at BL had functional ability comparable to population norms after 3 yrs; shorter disease duration and no prior DMARD/biologics use in this population suggests that earlier APR treatment may increase the likelihood of maximal functionality for some pts.
To cite this abstract in AMA style:
Mease PJ, Wells AF, Wollenhaupt J, Hall S, van Den Bosch F, Lespessailles E, McIlraith M, Nguyen D, Teng L, Edwards CJ. Long-Term Improvements in Physical Function of DMARD-Naive and DMARD/Biologic-Experienced Psoriatic Arthritis Patients Treated with Apremilast: Data from a Large Database of 4 Phase III Clinical Trials [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/long-term-improvements-in-physical-function-of-dmard-naive-and-dmardbiologic-experienced-psoriatic-arthritis-patients-treated-with-apremilast-data-from-a-large-database-of-4-phase-iii-clinical-trial/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-improvements-in-physical-function-of-dmard-naive-and-dmardbiologic-experienced-psoriatic-arthritis-patients-treated-with-apremilast-data-from-a-large-database-of-4-phase-iii-clinical-trial/