Session Information
Date: Wednesday, November 16, 2016
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment VI: Quality of Life
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder that causes long-term organ damage over time and impairment in health-related quality of life (HRQoL). We examined the long-term administration of belimumab plus standard of care (SoC) on HRQoL and fatigue in patients with autoantibody-positive SLE.
Methods: This multicenter continuation study (GSK study 112233, NCT00724867; Aug 2008–Mar 2015) enrolled patients who completed the BLISS-76 randomized controlled trial (RCT) in the US. Patients on belimumab in the RCT continued to receive the same dose (1 or 10 mg/kg IV, every 28 days; all 10 mg/kg post-Mar 2011) plus SoC (belimumab/belimumab group). Those who previously received SoC alone (the RCT placebo [PBO] group) received belimumab 10 mg/kg IV (PBO/belimumab group). Primary outcome measures included long-term safety and efficacy of belimumab, including HRQoL and fatigue, every 48 weeks including HRQoL and fatigue by Short Form-36 v2 (SF-36) Medical Outcomes Survey and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale, respectively. As the primary endpoint was safety, the baseline (BL) for this analysis was at start of belimumab administration.
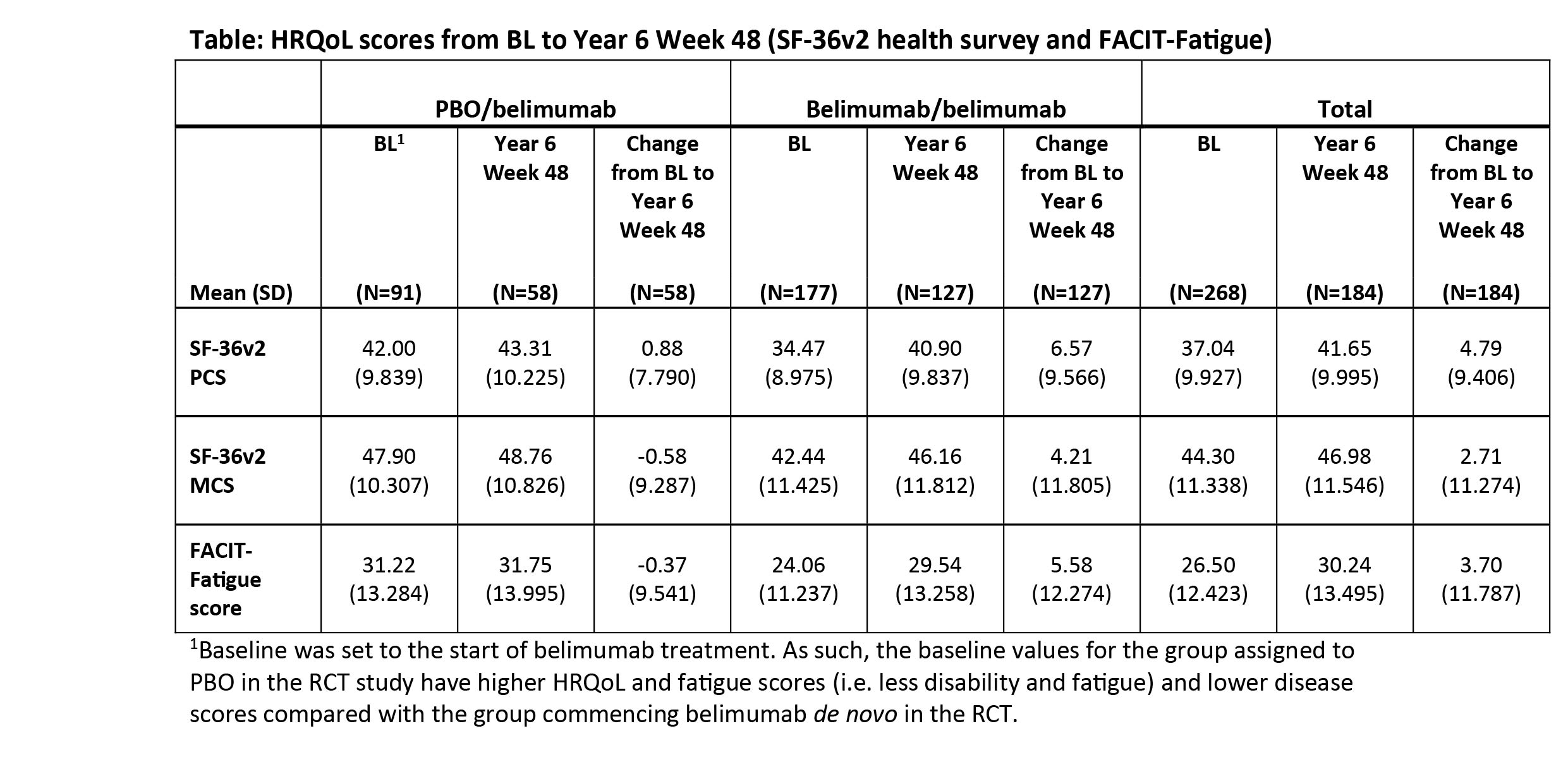
Results: The modified intent-to-treat population comprised 268 patients; 140 completed the continuation study, and 128 withdrew (patient request: n=31; adverse events: n=25). At BL, patients in the PBO/belimumab group reported higher SF-36 Physical (PCS) and Mental (MCS) Component, and FACIT scores than the belimumab/belimumab group (Table). Patients receiving long-term belimumab reported continued HRQoL and fatigue benefits, with improvements in SF-36 PCS, MCS and FACIT scores up to Year 6 Week 48 (Table). Mean changes from BL in the belimumab/belimumab group also exceeded minimum clinically important differences (MCID) at each yearly assessment to Year 6 (SF-36 PCS [range; 4.90–8.31], SF-36 MCS [range; 3.85–4.90], FACIT [range; 5.75–6.85]). At Year 6 Week 48, mean changes from BL in SF-36 domain scores exceeded MCID in 6 of 8 domains: bodily pain, general health, physical functioning, role physical, social functioning, and vitality.
Conclusion: Largest improvements in HRQoL and fatigue were reported in Year 1 of treatment, and maintained during long-term exposure to belimumab. Although reported improvements in the belimumab/belimumab group were greater than those in the PBO/belimumab group, these changes may have been confounded by differences in BL SF-36 scores, as well as SoC treatment with optimized medical care during the RCT. These data suggest that long-term control of disease activity with belimumab plus SoC translates into meaningful benefits in patient fatigue and HRQoL. Disclosure: Study funded by GSK and Human Genome Sciences, Inc. Nicole Cash, MRes PhD, Fishawack Indicia Ltd, UK, provided editorial assistance, funded by GSK.
To cite this abstract in AMA style:
Strand V, Berry P, Ramachandran S, Fettiplace J. Long-Term Impact of Belimumab on Health-Related Quality of Life and Fatigue in Patients with Systemic Lupus Erythematosus: Up to 7 Years of Treatment Exposure [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/long-term-impact-of-belimumab-on-health-related-quality-of-life-and-fatigue-in-patients-with-systemic-lupus-erythematosus-up-to-7-years-of-treatment-exposure/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-impact-of-belimumab-on-health-related-quality-of-life-and-fatigue-in-patients-with-systemic-lupus-erythematosus-up-to-7-years-of-treatment-exposure/