Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Tocilizumab (TCZ), a humanized anti–IL-6 receptor antibody, showed a favorable trend toward relapse suppression in patients (pts) with refractory Takayasu arteritis (TAK) in a randomized, double-blind (DB), placebo-controlled, multicenter trial in Japan (TAKT Study; Arthritis Rheumatol. 2016;68:suppl 10). However, little is known about the long-term efficacy and safety of TCZ in pts with TAK. Here we present the efficacy and safety of TCZ treatment over 52 weeks (wks) in the DB period and open-label extension (OLE) of the ongoing TAKT Study.
Methods: Pts ≥12 years old with TAK diagnosed according to the Japanese Circulation Society guideline (Circ J. 2011;75:474-503), who had relapsed while receiving oral glucocorticoid (GC; ≥0.2 mg/kg/day prednisolone equivalent) within the prior 12 wks, were randomly assigned 1:1 to weekly subcutaneous TCZ 162 mg or placebo (PBO) after achieving remission with oral GC therapy. During the DB period, background GC was tapered by 10%/wk from Wk 4 to a minimum of 0.1 mg/kg/day. Pts who experienced protocol-defined relapse during the DB period could enter the OLE period to receive weekly TCZ 162 mg. The DB period ended when relapse of TAK occurred in 19 pts and all pts were eligible to move on to the OLE period. During the OLE period, background GC doses were adjusted at the investigator’s discretion. All pts were evaluated for reduction of GC dose and protocol-defined relapse of TAK in long-term treatment of TCZ.
Results: Thirty-six pts were randomized to receive study treatment (18 pts received TCZ and 18 pts received PBO) in the DB period and all pts entered the OLE period. As of the November 2016 data cut-off, 7 pts had withdrawn from the study during the OLE period. The median (min–max) total duration of TCZ treatment was 70.4 (8.1–108.0) wks and 31 pts were treated with TCZ over 52 wks. The median GC dose decreased from 0.22 mg/kg/day at relapse before participation in the study to 0.13 mg/kg/day at Wk 52 (Figure). Twelve of the 31 pts achieved at least 50% reduction of GC dose and 2 pts were weaned off GC at Wk 52. Protocol-defined relapse was observed in 8 pts during the OLE period. The exposure-adjusted relapse rate was 23.6 events per 100 patient-years (PYs) in the OLE period while they were 203.1 events per 100 PYs in the PBO group and 101.1 events per 100 PYs in the TCZ group in the DB period. In total, 34 (94.4%) pts experienced at least 1 adverse event (AE) while receiving TCZ. The exposure-adjusted AE rate did not increase after the DB period. Serious AEs were reported in 6 pts (16.7%). Infections were the most frequent AEs (31 pts; 86.1%) and SAEs (2 pts; 5.6%). No new safety concerns were observed with TCZ throughout the study. No pts died in this study.
Conclusion: Over 52 wks, TCZ exhibited sustained, clinically meaningful, steroid-sparing effects in pts with refractory TAK. TCZ continued to show a safety profile consistent with that observed with RA/JIA.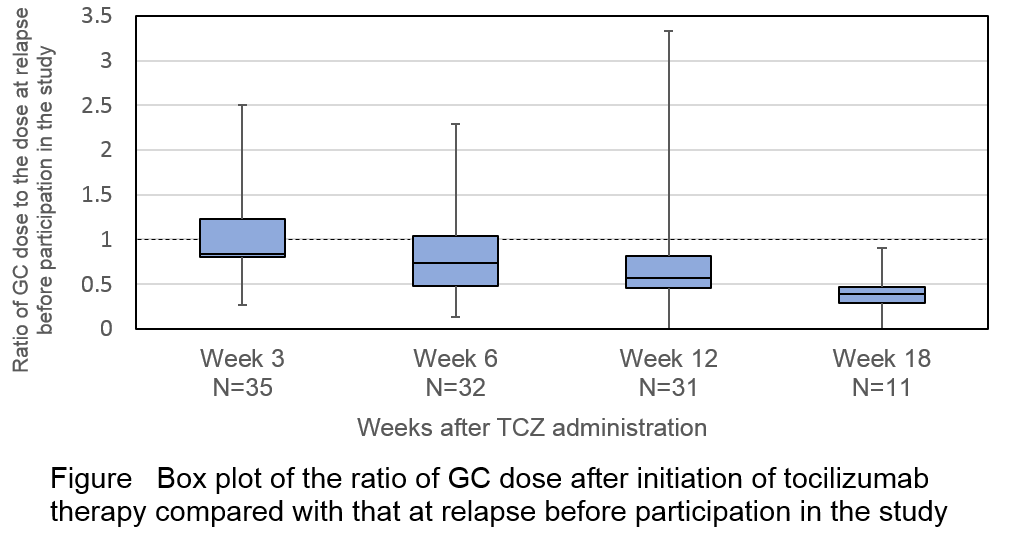
To cite this abstract in AMA style:
Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, Nomura A, Yoshida S, Nishimoto N. Long-Term Efficacy and Safety of Tocilizumab in Patients with Refractory Takayasu Arteritis Treated Continuously over 52 Weeks: Results from Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial and Open-Label Extension in Japan [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-tocilizumab-in-patients-with-refractory-takayasu-arteritis-treated-continuously-over-52-weeks-results-from-phase-3-randomized-double-blind-placebo-controlled-trial/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-tocilizumab-in-patients-with-refractory-takayasu-arteritis-treated-continuously-over-52-weeks-results-from-phase-3-randomized-double-blind-placebo-controlled-trial/
