Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Risankizumab (RZB), a humanized immunoglobulin G1 monoclonal antibody that inhibits interleukin-23 by targeting its p19 subunit with high affinity and specificity, is approved for the treatment of adult patients (pts) with active PsA. Pts in the KEEPsAKE 1 trial who received RZB 150 mg achieved the primary efficacy endpoint at week 24. The long-term efficacy, safety and tolerability of RZB has been previously reported through 100 weeks of treatment. Here, we report the efficacy and safety results through week 148.
Methods: KEEPsAKE 1 is an ongoing, global, phase 3, multicenter clinical trial to evaluate the efficacy and safety of RZB versus placebo (PBO) in patients with active PsA. Eligible patients were≥18 years old and demonstrated an inadequate response, intolerance or contraindication to ≥1 conventional syntheticDMARD (csDMARD-IR). Following a 24-week double-blind, PBO-controlled, parallel-group treatment period (period 1), the trial is ongoing with an open-label extension treatment period from week 24 through week 316 (period 2). In period 1, pts were randomized 1:1 to receive subcutaneous RZB 150 mg or PBO at weeks 0, 4 and 16. At week 24, pts who were randomized to RZB received blinded PBO and pts who were randomized to PBO received the first dose of blinded RZB. Starting at week 28, all pts continue to receive open-label RZB 150 mg every 12 weeks until week 316. Beginning at week 36, pts who were classified as non-responders (defined as < 20% improvement in tender or swollen joint count on two consecutive visits vs baseline) were discontinued from the study drug. Efficacy and safety analyses were conducted in all randomized pts who received one or more doses of the study drug. Safety assessments were based on monitoring of treatment-emergent adverse events (TEAEs).
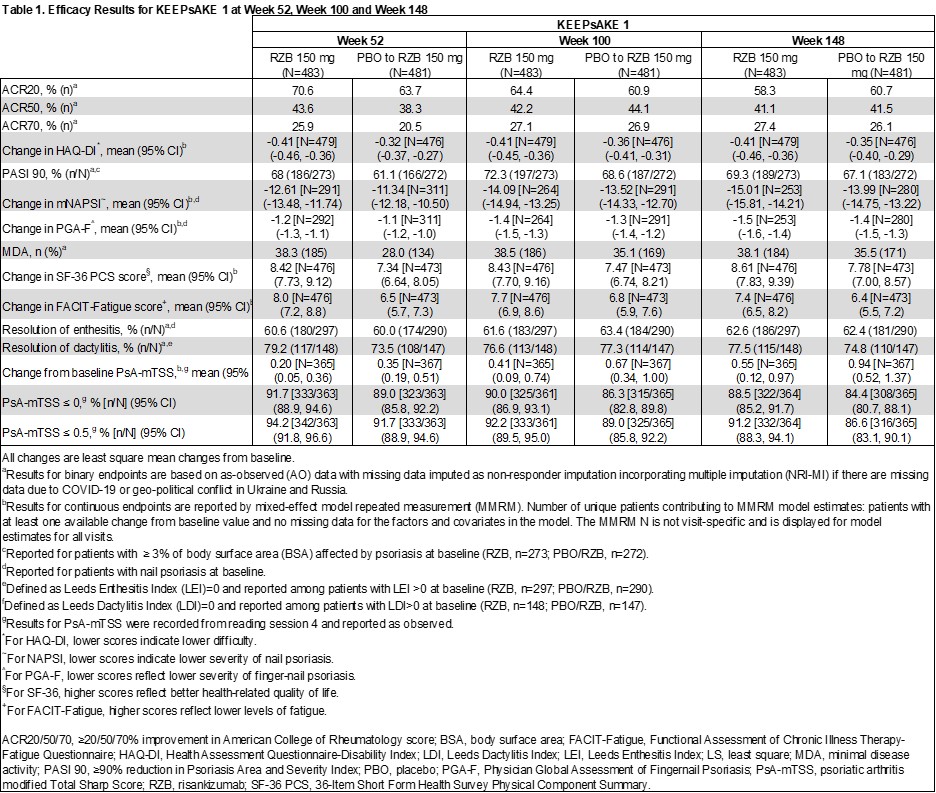
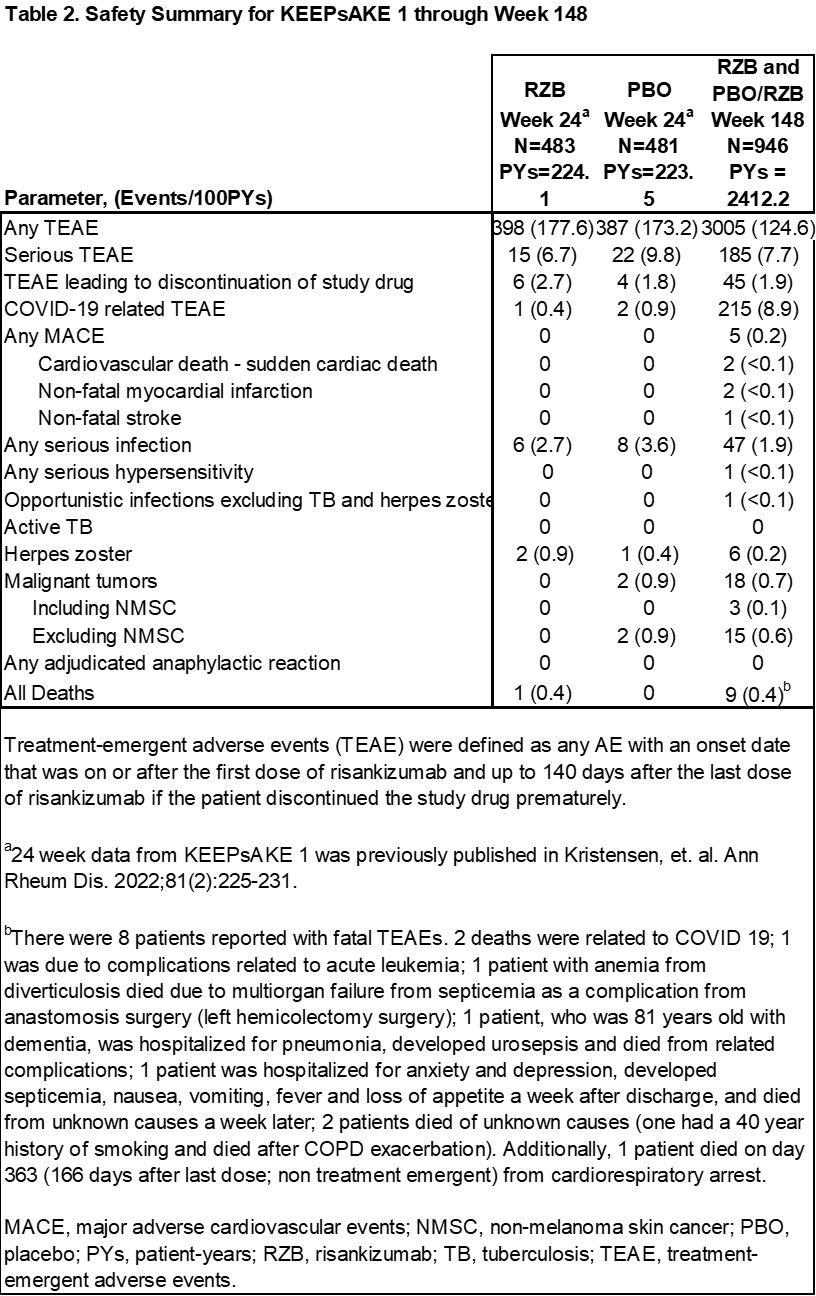
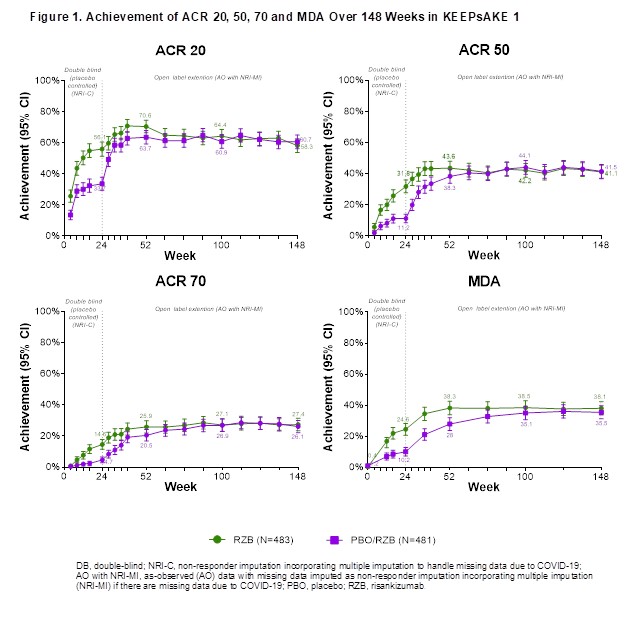
Results: Overall efficacy results were maintained at week 148 of the KEEPsAKE 1 trial, as compared to week 52 and 100 (Table 1). At week 148, 41.1% of RZB and 41.5% of PBO/RZB pts achieved ACR50 and 38.1% of RZB and 35.5% of PBO/RZB pts achieved MDA (Figure 1). 69.3% of RZB and 67.1% of PBO/RZB pts achieved PASI90 at week 148. mNAPSI and PGA-F scores improved from baseline by 15.01 and 1.5 points, respectively, for RZB pts and by 13.99 and 1.4 points for PBO/RZB pts. For pts with enthesitis at baseline, resolution was seen in 62.6% of RZB and 62.4% of PBO/RZB pts. For pts with dactylitis at baseline, resolution was seen in 77.5% of RZB and 74.8% of PBO/RZB pts. At week 148, the mean PSA-mTSS score increased by 0.55 from baseline for RZB and by 0.94 for PBO/RZB pts. 88.5% of RZB and 84.4% of PBO/RZB pts showed no radiographic progression (PSA-mTSS ≤0 from baseline). At week 148, HAQ-DI (RZB -0.41, PBO/RZB -0.35), SF-36 PCS (RZB 8.61, PBO/RZB 7.78) and FACIT-Fatigue (RZB 7.4, PBO/RZB 6.4) scores showed consistent increase from baseline. The overall rates of TEAEs, serious TEAEs and AEs leading to discontinuation of study drug have remained stable and comparable to those reported in period 1 (Table 2).
Conclusion: The 148-week results of the ongoing KEEPsAKE 1 trial demonstrate the durable efficacy of RZB 150 mg in treating the clinical manifestations and improving health-related quality of life in csDMARD-IR pts with PsA. RZB was well-tolerated, with no new safety signals.
To cite this abstract in AMA style:
Erik L, Keiserman M, Papp K, McCasland L, White D, Stakias V, Iyile T, Carter K, Soliman A, Drogaris L, Chen M, Padilla B, Behrens F. Long-Term Efficacy and Safety of Risankizumab for CsDMARD-IR Patients with Active Psoriatic Arthritis: 148-Week Results from the KEEPsAKE 1 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-risankizumab-for-csdmard-ir-patients-with-active-psoriatic-arthritis-148-week-results-from-the-keepsake-1-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-risankizumab-for-csdmard-ir-patients-with-active-psoriatic-arthritis-148-week-results-from-the-keepsake-1-trial/