Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: PsA is a chronic systemic inflammatory disease that affects 30% of patients diagnosed with psoriasis, with a clinical burden that includes dactylitis, enthesitis, cutaneous manifestations, chronic pain, progressive joint damage, and disability. Risankizumab (RZB), an antibody that targets the p19 subunit of interleukin-23 with high affinity and specificity, is approved for the treatment of adult patients with active PsA. RZB has been previously reported to show efficacy across several disease domains up to week 100.1 Here, we report the efficacy and safety results through week 148.
Methods: KEEPsAKE 2 is an ongoing phase 3 global, multicenter clinical trial evaluating the efficacy and safety of RZB versus placebo (PBO) in patients with active PsA,defined as ≥5 tender joints and ≥5 swollen joints, meeting the Classification Criteria for Psoriatic Arthritis (CASPAR), with symptom onset of ≥6 months before screening, and active plaque psoriasis or nail changes consistent with psoriasis at screening. Eligible patients were 18 years or older and had previous inadequate response or intolerance to 1 or 2 biological therapies (Bio-IR) and/or ≥1 conventional synthetic DMARD (csDMARD-IR). Patients were randomized in a 1:1 ratio to receive double-blinded treatment with subcutaneous RZB 150 mg or matched placebo for 24 weeks, administered at weeks 0, 4 and 16. Starting at week 24, all patients in the ongoing trial receive open-label RZB 150 mg every 12 weeks through week 316. Efficacy and safety analyses were conducted in all randomized patients who received one or more doses of the study drug. Statistical reporting and imputation methods for efficacy assessments are defined in the figures. Safety assessments were based on monitoring of treatment emergent adverse events (TEAEs) and are summarized using exposure-adjusted event rates (EAERs, events/100 patient-years [PYs]).
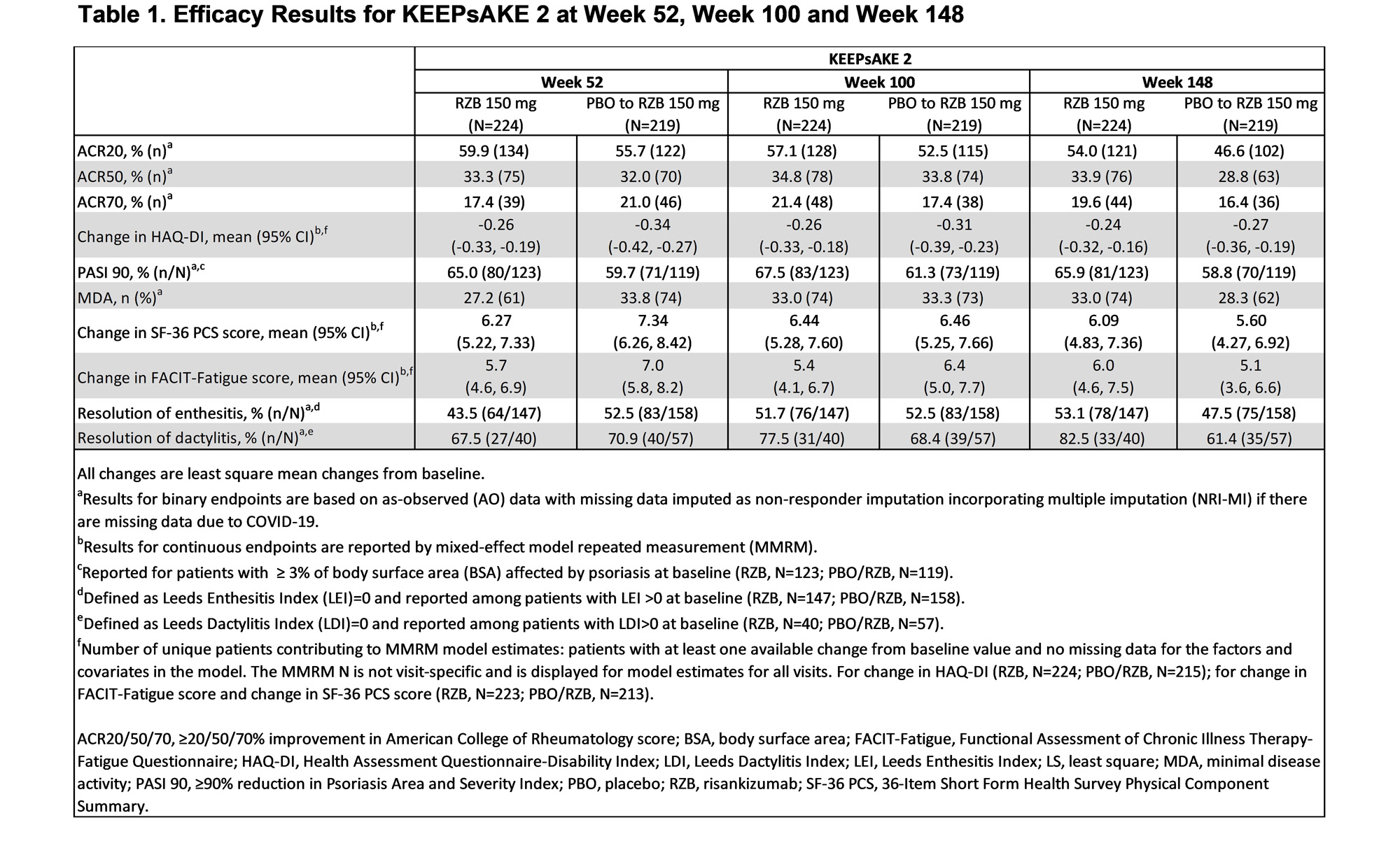
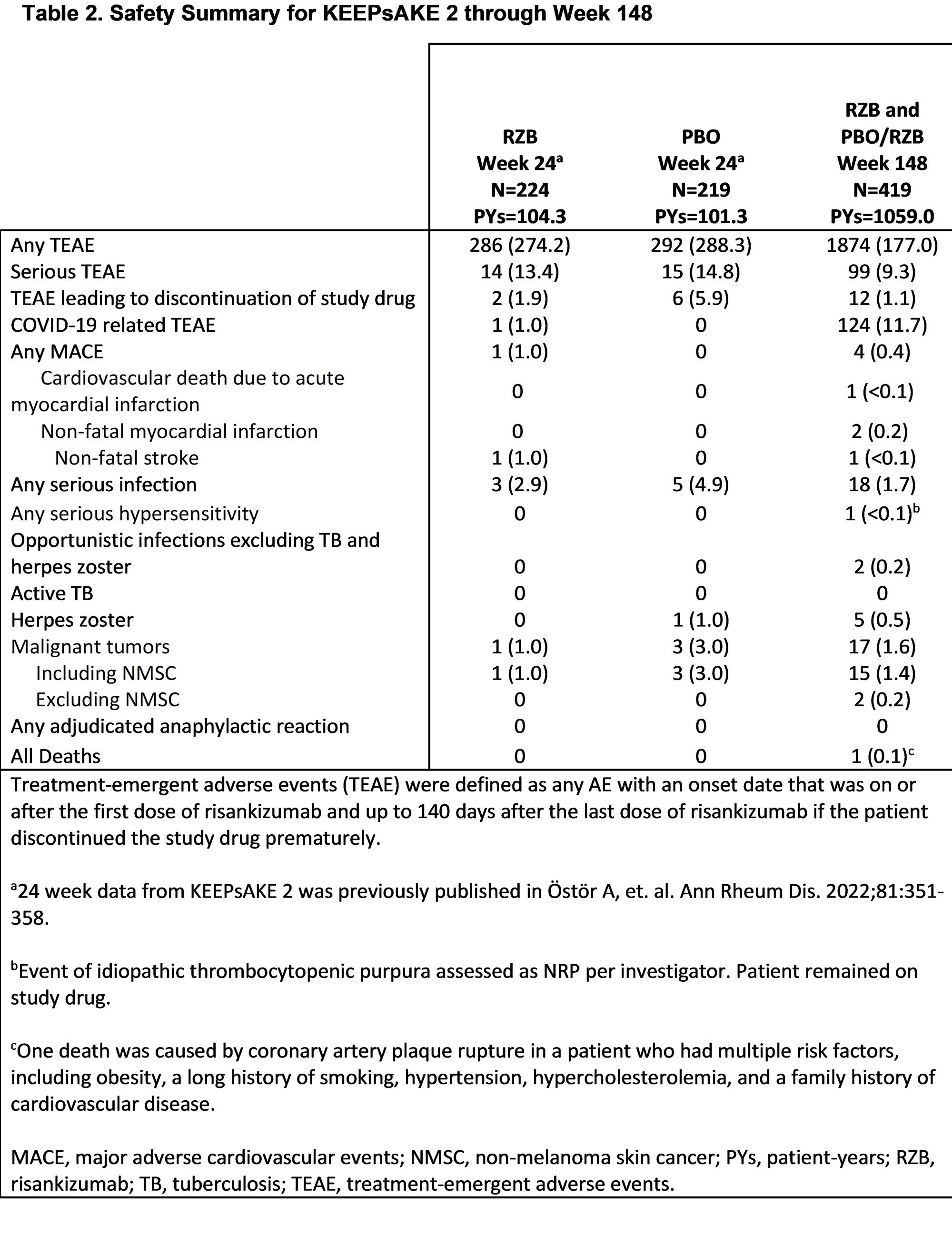
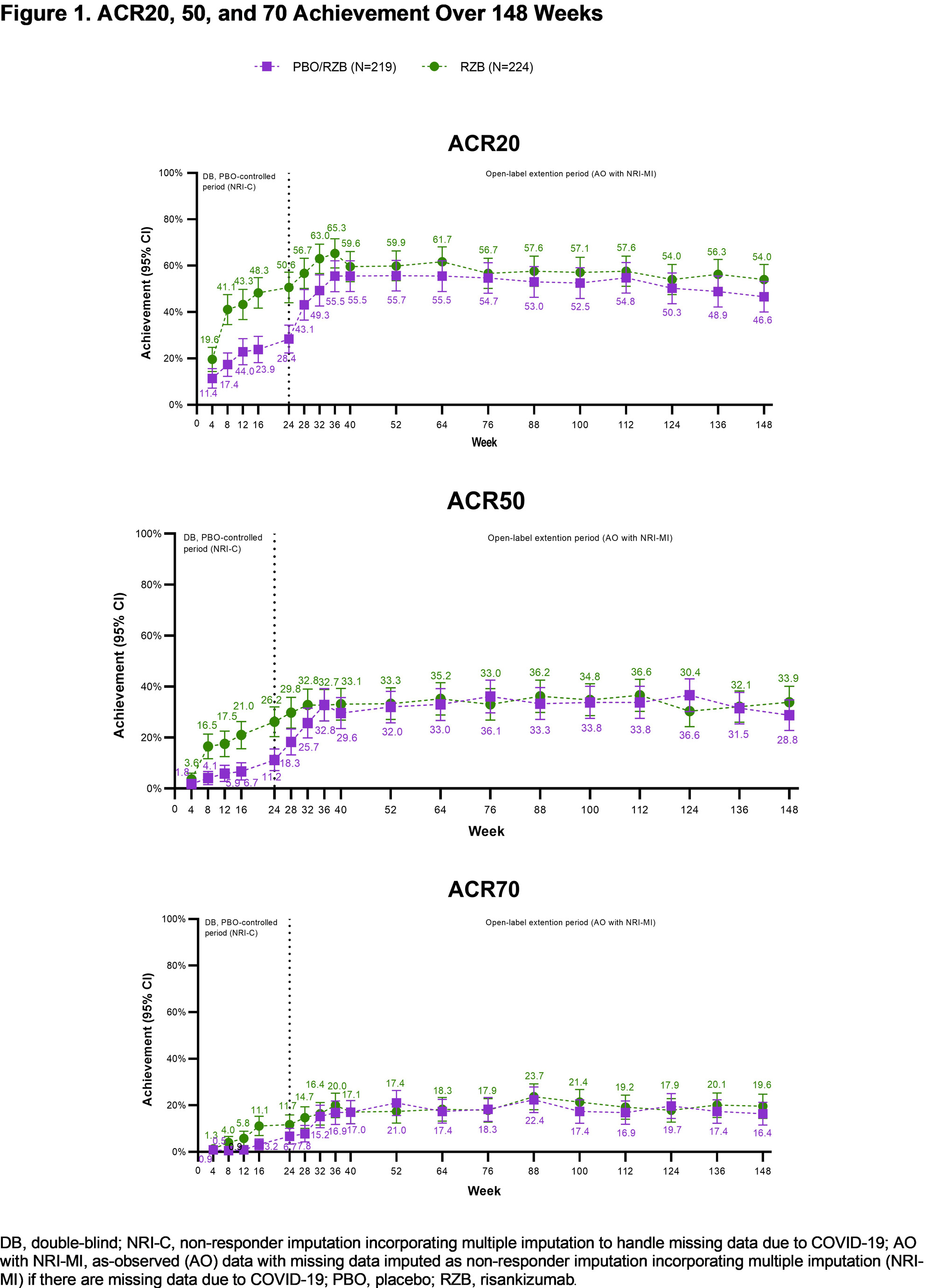
Results: Patients in KEEPsAKE 2 (RZB N=224; PBO/RZB N=219) maintained similar efficacy results at week 148 to those reported at week 52 and week 100 (Table 1). 33.9% of RZB and 28.8% of PBO/RZB patients achieved ACR50 response at week 148 (Figure 1). 65.9% of RZB and 58.8% of PBO/RZB patients achieved PASI 90 response at week 148. A consistent change from baseline in HAQ-DI (RZB -0.24, PBO/RZB -0.27), SF-36 PCS (RZB 6.09, PBO/RZB 5.60) and FACIT-Fatigue (RZB 6.0, PBO/RZB 5.1) was observed at 148 weeks. 33.0% of RZB patients and 28.3% of PBO/RZB patients achieved MDA at week 148, consistent with results reported at week 52 and week 100. For those patients with enthesitis at baseline, resolution was observed in 53.1% of RZB and 47.5% of PBO/RZB patients at week 148. For patients with dactylitis at baseline, resolution was observed in 82.5% of RZB and 61.4% of RZB/PBO patients at week 148. The overall rates of TEAEs, serious TEAEs and AEs leading to discontinuation of study drug remained stable and was consistent with the rates reported for the placebo-controlled period (Table 2).
Conclusion: Long-term treatment with RZB 150mg shows durable efficacy in patients with PsA through 148 weeks, with no new safety findings.
References:
- Kristensen, et al. 2022 EADV Congress.
To cite this abstract in AMA style:
Östör A, Van den Bosch F, Papp K, Asnal C, Blanco R, Aelion J, Stakias V, Iyile T, Carter K, Soliman A, Drogaris L, Chen M, Padilla B, Kivitz A. Long-Term Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 148-Week Results from the KEEPsAKE 2 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-risankizumab-for-active-psoriatic-arthritis-148-week-results-from-the-keepsake-2-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-risankizumab-for-active-psoriatic-arthritis-148-week-results-from-the-keepsake-2-trial/