Session Information
Date: Tuesday, November 12, 2019
Title: Pediatric Rheumatology – ePoster III: Systemic JIA, Fever, & Vasculitis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Canakinumab (CAN) is an efficacious option for treatment of systemic juvenile idiopathic arthritis (sJIA). However, it is still disputable whether long-term therapy is efficacious and drug-free remission can be achieved. This study aimed to analyze the efficacy and safety of long-term CAN treatment in children.
Methods: Nineteen patients aged 2.3–15.6 years who had started CAN therapy under CACZ885G2306 clinical trial were enrolled in this study. The patients’ median age at treatment initiation was 9.6 (IQR 6.4–11.1) years; age at sJIA onset was 3.2 (1.9–5.1) years; and sJIA duration was 4.4 (1.2–7.0) years. Before the initiation of CAN therapy, two (10.5%) patients were biologic-naïve; 12 (63.2%) patients had received one prior biologic agent; and 5 (26.3%) patients had received at least two prior biologic agents. All patients had been earlier treated with NSAIDs; 16 (84.2%) patients had been receiving glucocorticoids; 15 (78.9%), methotrexate; 10 (52.6%), cyclosporine A; and one (5.3%) patient, sulfasalazine. Response to therapy was assessed using the ACRPedi 30/50/70/90 criteria and the C. Wallace criteria for clinical remission. Safety was assessed at each visit
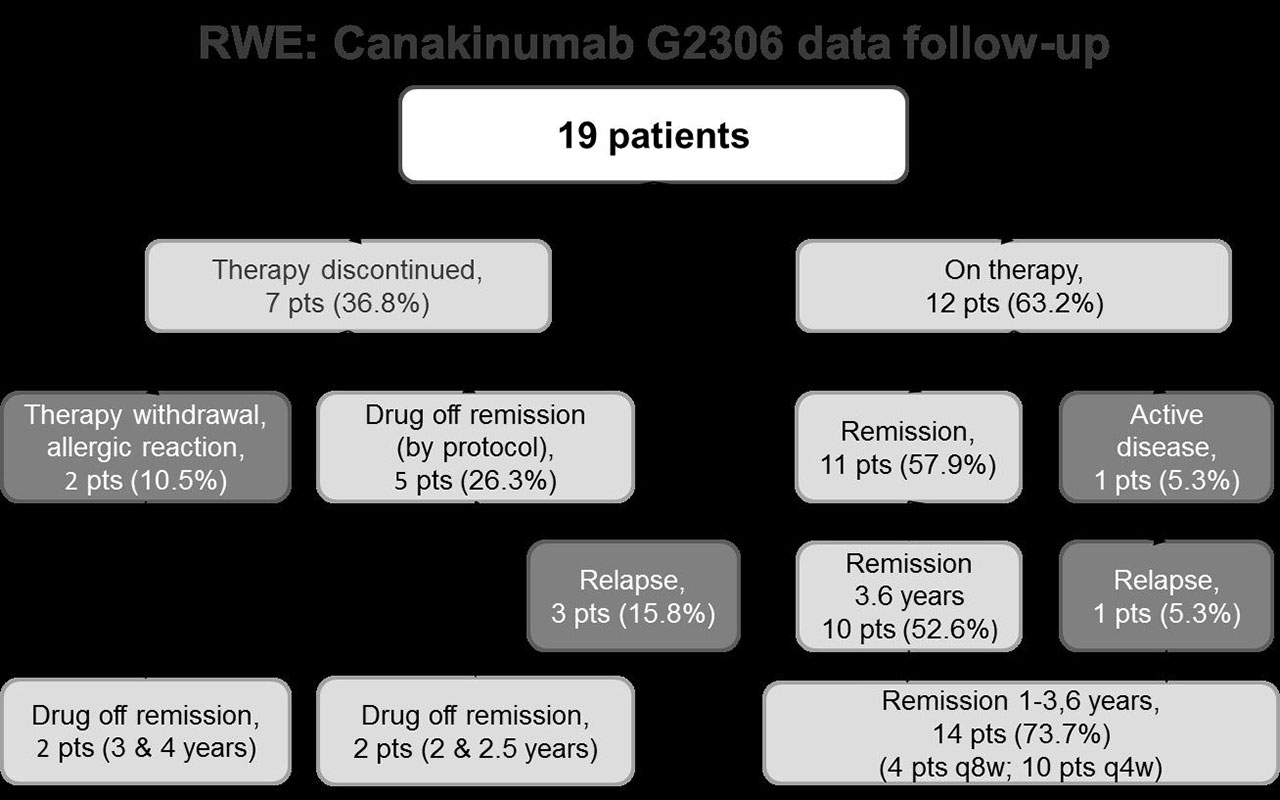
Results: At treatment initiation, disease activity was high in all patients. Depending on the criteria used for evaluation, JIA activity was as follows: 14.7 (12.2–25.3) for JADAS-71; 41 (36.5-69) for physician’s global assessment VAS; 54 (45-79.5) for patient’s global assessment VAS; and 0.5 (0.1-0.8) for the CHAQ disability index. After 12-month treatment, 14 (73.7%) patients attained inactive disease according to the C. Wallace’s criteria. JADAS-71 decreased by 14.5 (11-25.4) points (p< 0.001 compared to baseline). Fourteen (73.7%) patients achieved an ACR100 response. The median treatment duration was 42.6 (39.5-72.5) months; total treatment duration was 79.2 patient-years. Figure shows the treatment outcomes and the reasons for therapy discontinuation.
One of 11 patients remaining in therapy developed a relapse accompanied by a MAS episode that required elevation of the GC dose. CAN therapy was continued, and the patient achieved remission again. Fourteen (73.7%) of 19 patients receiving CAN therapy achieved remission lasting 1–3.6 years; the interval of CAN administration was increased to 8 weeks in four patients.
Four (21.1%) patients achieved drug-free remission and had no relapses over the follow-up period of 2-4 years.
During the follow-up period, serious adverse events (SAE) were reported in two (6.1%) patients (severe allergic reaction – 1 and enteritis – 1). Five (10.2%) patients had AEs (allergy – 1; hepatotoxicity – 2; exacerbation of herpes infection –1; and neutropenia – 1).
Conclusion: Long-term CAN therapy proved to be efficacious and safe; patients remain in remission after medication discontinuation.
To cite this abstract in AMA style:
Alexeeva E, Denisova R, Dvoryakovskaya T, Isaeva K, Kriulin I, Alshevskaya A, Moskalev A. Long-Term Efficacy and Safety of Сanakinumab in Children with Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-%d1%81anakinumab-in-children-with-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-efficacy-and-safety-of-%d1%81anakinumab-in-children-with-systemic-juvenile-idiopathic-arthritis/