Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Certolizumab pegol (CZP) monotherapy administered every 4 weeks (Q4W) for rheumatoid arthritis (RA) has been shown to be associated with rapid and sustainable improvements in productivity up to 2 years.1 The current analysis evaluates the long-term impact on household productivity and social participation of CZP 400mg Q4W combination and monotherapy over 5 years.
Methods: In this open-label extension (OLE) (NCT00160693) patients (pts) originally enrolled in FAST4WARD (NCT00548834) or study 014 (NCT00544154) received CZP 400 mg Q4W for 24 weeks (wks). Pts who completed or withdrew on/after Wk12 in either study were eligible and were permitted to take DMARDs in OLE. Household productivity and social participation were assessed through the RA-specific Work Productivity Survey (WPS-RA); results are reported up to Wk268 (5.2yrs). The analyzed population consisted of (1) Wk 24 CZP completers from FAST4WARD or 014 who entered the OLE (all pts group) and (2) CZP completers from FAST4WARD who entered OLE and did not receive MTX or another DMARD at any point (monotherapy subgroup).
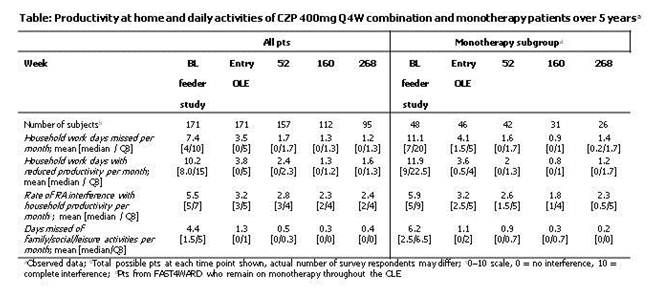
Results: From the CZP groups of the FAST4WARD (N=111) and 014 (N=124) feeder studies, 75 and 96 pts, respectively, completed the studies and entered the OLE. 48 pts who completed FAST4WARD and entered the OLE remained on monotherapy throughout their time in the OLE, and of these 26 remained in OLE at Wk268. In both populations analyzed, a rapid reduction in the number of days missed of household work per month was seen from feeder study baseline (BL, 7.4 and 11.1 mean days respectively) over 24wks of the feeder studies to OLE entry (3.5 and 4.1 mean days respectively) and continued to decline over time, up to Week 268 (1.2 and 1.4 mean days respectively; Table). A similar decrease was reported in the number of household days with reduced productivity per month, and in the rate of RA interference with household productivity per month (Table). Increased participation in family/social/leisure activities was reported in both populations, with a decrease in the number of days missed per month from feeder study baseline (4.4 and 6.2 mean days, respectively), to entry to OLE, at a mean of 1.3 and 1.1 days respectively for the 2 groups; these improvements continued over the 5 years to 0.4 and 0.2 days on average in the 2 populations respectively.
Conclusion: CZP treatment, in combination with MTX/DMARDs or as monotherapy, improved household productivity and increased participation in social/family/leisure activities, as shown by the decrease in the number of household work or social/family/leisure days missed or the number of days with reduced productivity per month. These improvements were maintained up to 5 years with open-label CZP following 24 wks double-blind CZP therapy.
References: 1. Strand V et al. Ann Rheum Dis. 2011;70(6):996-1002
Disclosure:
V. Strand,
UCB,
5;
O. Purcaru,
UCB,
3;
R. F. van Vollenhoven,
UCB,
2,
UCB,
5;
E. Choy,
UCB,
2,
UCB,
5,
UCB,
8;
R. Fleischmann,
UCB,
2,
UCB,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-benefits-of-4-weekly-certolizumab-pegol-combination-and-monotherapy-on-household-productivity-and-social-participation-in-rheumatoid-arthritis-5-year-results-from-an-open-label-extension-st/