Session Information
Date: Tuesday, October 23, 2018
Title: 5T093 ACR Abstract: Vasculitis–Non-ANCA-Assocd & Rel D/Os II: Novel Diagnostics & THRs (2838–2843)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose:
LEF could be the favourable DMARD for PMR/GCA due to inhibitory activity on dendritic cells and IL-6. Previous case series have shown efficacy of LEF in PMR and GCA(1). Higher risk of adverse events due to CS use and disease activity is most associated in patients with relapsing/refractory disease. In current biologic era, LEF could be an efficacious and cost-effective treatment alongside with low dose of CS(2).
Methods:
We conducted a retrospective cohort study to evaluate the efficacy and safety profile of LEF in 40 patients with GCA and 23 with PMR. Information was collected from our database of all patients with known GCA and PMR. Demographics, baseline characteristics, prednisolone dose, CRP values, disease and treatment duration with LEF and side effects were reviewed.
Results:
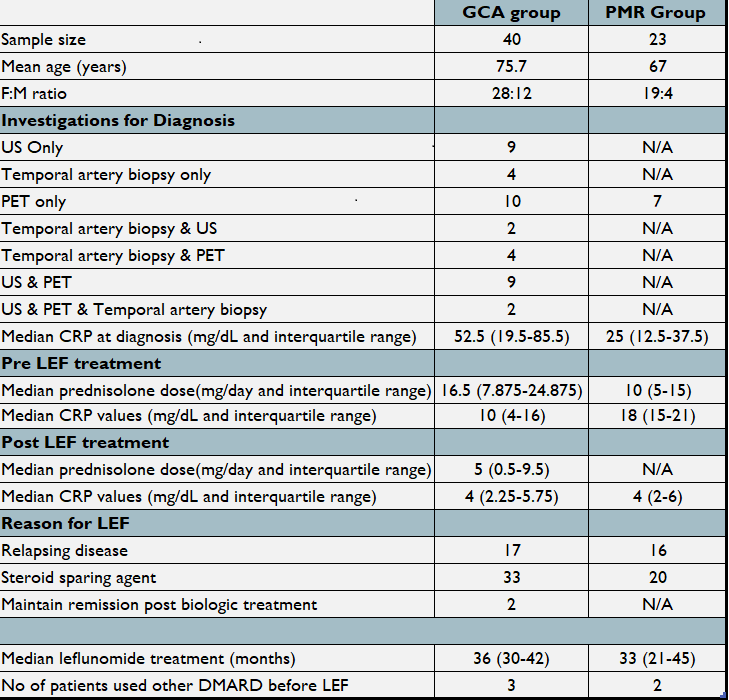
Baseline characteristics, investigations, CRP, median treatment duration of CS and LEF are summarised in Image 001. A Wilcoxon signed rank test was used. For 30 GCA and 16 PMR patients (10 and 7 CRP missing respectively, excluded from analysis), median decrease of CRP on initiation and 6 months post LEF treatment was statistically significant (z=48.5, p<0.01 and z=7.5, p=0.13 respectively). The median reduction in prednisolone dose from initiation to 6 months was also statistically significant (z=44.5, p<0.000). Median time to reduce Prednisolone to <5mg/day was 5.5 months (achieved in 17/23) in the PMR group and 6.5 months for the GCA group (achieved in 9/16) since starting on LEF.
Hypertension, hair thinning, rash, gastrointestinal disturbances, liver function test abnormalities and infections were reported in 15 patients in the GCA group. Rash, nausea, weight loss and gastrointestinal disturbances were reported in 7 patients in the PMR group. LEF was discontinued in 7/40 patients in GCA group; 3 due to side effects, 1 due to diagnosis of lymphoma, 1 due to pneumonia which required short admission to the hospital, 2 due to flare which required steroids dose increase despite leflunomide and 1 due to peripheral neuropathy. LEF was discontinued in 7/23 in PMR group due to side effects. One death reported in a patient who was started on LEF 3 months earlier; cause is unknown. In 3/63 patients LEF was discontinued as they sustained remission.
Conclusion:
LEF seems to be effective as a CS sparing agent in patients with GCA and PMR. Randomized controlled trials are warranted to confirm the usefulness of leflunomide in the therapy of GCA/PMR.
References:
1. Adizie T, et al. Efficacy and tolerability of leflunomide in difficult-to-treat polymyalgia rheumatica and giant cell arteritis: a case series. Int J Clin Pract. 2012 Sep;66(9):906–9.
2. Strehl C, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis. 2016 Jun;75(6):952–7.
To cite this abstract in AMA style:
Laskou F, Coath F, Sidhu A, Wahid A, Dasgupta B. Leflunomide in Giant Cell Arteritis and Polymyalgia Rheumatica: A Real World Single Centre Experience [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/leflunomide-in-giant-cell-arteritis-and-polymyalgia-rheumatica-a-real-world-single-centre-experience/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/leflunomide-in-giant-cell-arteritis-and-polymyalgia-rheumatica-a-real-world-single-centre-experience/
