Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tuberculosis (TB) testing prior to biologic DMARD use helps prevent potentially fatal, medication-related adverse events and is a National Quality Forum-endorsed quality measure. We used data from a national registry to describe practice variations on this measure among US rheumatologists.
Methods: The ACR’s RISE is a national registry that passively collects electronic health record (EHR) data on all patients seen by participating practices. As of December 2016, RISE was connected to 632 providers representing an estimated 20% of the US clinical rheumatology workforce. We calculated the proportion patients ≥ 18 with rheumatoid arthritis who were newly prescribed a biologic DMARD or tofacitinib during 2016 and who had TB testing in the 12 months preceding the biologic prescription, by practice and geographic region. We examined associated EHR data for the type of TB test performed (PPD or quantiferon-based assays). Consistent with other national performance analyses, providers with fewer than 30 qualifying patients in the denominator were excluded. Proportions were compared using chi-square tests.
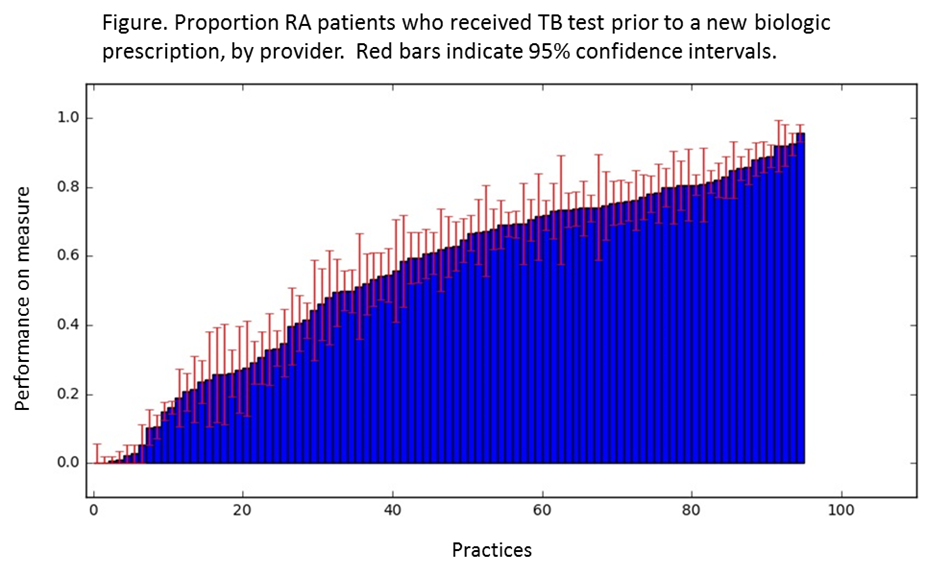
Results: We analyzed data from 18,079 patients across 533 providers and 95 practices. 77% of patients were female, 20% were non-white, and mean age was 58 (SD 13.5). Overall, patient-level performance on the measure was 56%, with a range of 0 to 96% across practices (Figure), and significant variation across regions (Table; p<0.0001). Among the patients in the numerator with a TB test found in the EHR data (N=7828), 93% received a Quantiferon test while 7% had a PPD.
Conclusion: We found large variations in TB testing prior to new biologic DMARD starts across RISE practices. Given that quantiferon-based assays represented the majority of TB screening tests identified, we suspect that PPD testing remains undercaptured in the EHR. As value-based incentive programs become more widespread, accurate capture of performance on patient safety measures will be increasingly important and may require practices to standardize documentation of important safety processes such as TB testing.
Disclaimer: The data presented here was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors and do not necessarily represent the views of the ACR.
To cite this abstract in AMA style:
Schmajuk G, Evans M, Kay J, Yazdany J. Large Variations in Tuberculosis Testing for New Biologic Users with Rheumatoid Arthritis Among Providers in the RISE Registry [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/large-variations-in-tuberculosis-testing-for-new-biologic-users-with-rheumatoid-arthritis-among-providers-in-the-rise-registry/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/large-variations-in-tuberculosis-testing-for-new-biologic-users-with-rheumatoid-arthritis-among-providers-in-the-rise-registry/