Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Participant diversity in clinical trials of therapeutics in rheumatology is important to evaluate to better understand how individuals of different races and ethnicities might respond differently, if at all, to therapeutics. Specifically, for psoriatic arthritis (PsA), although people of color (POC) have lower disease prevalence, it still ranges from 0.04-0.19% in Blacks and 0.09-0.30% in Hispanics versus 0.19-0.34% in Whites in the insured population of the US1. Literature on the diversity of PsA clinical trials and the therapeutic effects on individuals of POC remains limited. This analysis aims to describe the reporting and racial/ethnic distribution of participants in clinical trials of US Food and Drug Administration approved targeted therapies for PsA.
Methods: Targeted therapies approved for use in the treatment of PsA in the US were identified. Package inserts and ClinicalTrials.gov (CT.gov) were used to determine the pivotal double-blind, randomized clinical trials (RCTs) which supported the approval of the therapeutics for PsA in the US, and the articles reporting the primary endpoint data were reviewed. Race and ethnicity data were extracted from the published data. Countries in which the studies were conducted were identified from the publications or CT.gov.
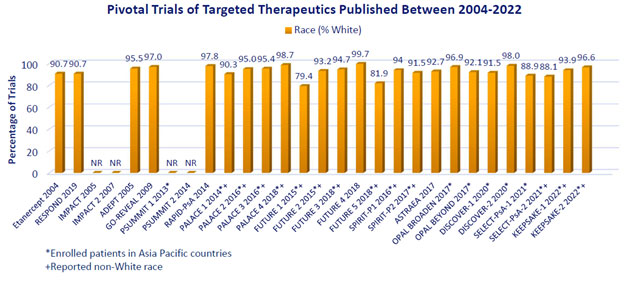
Results: Twenty-nine pivotal RCTs of targeted therapeutics published from 2002 – 2022 were identified; 25 reported race; individuals of non-White race were reported in only 13. In the latter, people of Black race comprised < 1% of the overall population in 12 RCTs and 2.7% in the remaining RCT. People of Asian race comprised < 10% in 11 studies and 11.3% and 19.0% in the remaining 2 studies, though 20 trials occurred in Asia Pacific countries (Figure). Hispanic/Latinx ethnicity was not reported in any study. Later studies (2017-2022) reported non-White race (n=7 of 15 [47%]) no more frequently than studies from 2004-2016 (n=6 of 14 [43%]). Although the 13 RCTs reporting non-White race may not reflect unique individuals, the total number of people studied was 7261, of which 48 (0.7%), 434 (6.0%), and 6598 (90.9%) were Black, Asian, and White, respectively.
Conclusion: These data show continued under-reporting of race and ethnicity in publications of PsA clinical trials. Even when reported, compared to the overall US population of which 72% and 62% were non-Hispanic Whites in 2010 and 2020, respectively, non-Hispanic Whites were over-represented in pivotal trials of PsA. This over-representation is notable when considering the prevalence of PsA by race in the US or that individuals of different races/ethnicity may have been less prevalent in other countries where recruitment occurred. Efforts to increase the reporting of race/ethnicity and ensure adequate representation of racial/ethnic minorities in PsA clinical trials are needed.
References:
1. Ogdie A, et al. Rheumatol Ther. 2021;8(4):1725-39.
2. https://trialfacts.com/diversity-inclusion/ accessed 22 May 2022
To cite this abstract in AMA style:
Goel N. Lack of Racial and Ethnic Diversity in Clinical Trials of Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/lack-of-racial-and-ethnic-diversity-in-clinical-trials-of-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lack-of-racial-and-ethnic-diversity-in-clinical-trials-of-psoriatic-arthritis/