Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral JAK inhibitor for the treatment of rheumatoid arthritis (RA). Treatment guidelines recommend targeting remission or low disease activity (LDA), herein defined as Disease Activity Scores ≤3.2 based on 28 joint counts (DAS28≤3.2) and adjusting therapy after 3–6 months1 (Mos). Predicting the likelihood of clinical response may help inform early treatment decisions. The aim of this study was to understand the relationship between timing and magnitude of early changes in DAS28, erythrocyte sedimentation rate (DAS28-4[ESR]) and the likelihood of achieving LDA at Mo 6 in RA patients (pts) naïve to methotrexate (MTX).
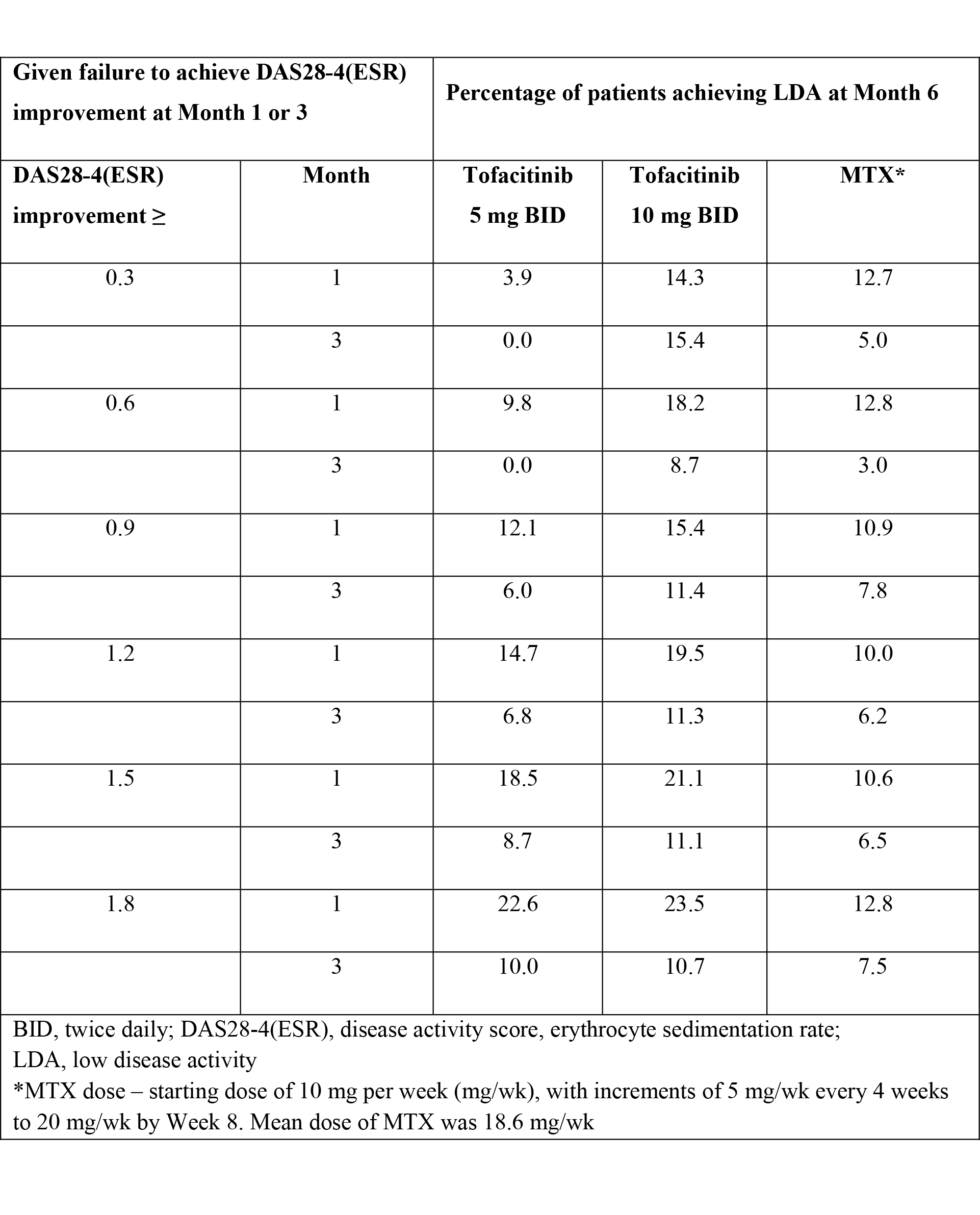
Methods: In a Phase 3 study (ORAL Start; NCT01039688), MTX-naïve RA pts were randomized 2:2:1 to the following monotherapies: tofacitinib 5 mg twice daily (BID), tofacitinib 10 mg BID or MTX. Conditional probabilities of pts achieving LDA at Mo 6 were calculated, given failure to achieve DAS28-4(ESR) improvement from baseline (range ≥0.3 to 1.8) at Mo 1 or 3. One-year data with non-responder imputation for missing values was used.
Results: 948 pts received treatment; tofacitinib 5 mg BID (n=370), tofacitinib 10 mg BID (n=394) or MTX (n=184); results previously reported.2 In this post-hoc analysis, of those patients who failed to achieve DAS28-4(ESR) improvement from baseline ≥1.2 at Mo 3, 6.8% for tofacitinib 5 mg BID, 11.3% for tofacitinib 10 mg BID, and 6.2% for MTX achieved LDA at Mo 6 (Table). A minority of pts (74/353 [20.9%]) receiving tofacitinib 5 mg BID failed to achieve DAS28-4(ESR) improvement ≥1.2 from baseline at Mo 3, while a greater proportion failed for MTX (65/171 [38.0%]). Failure to achieve DAS28-4(ESR) improvement from baseline at Mo 3 (range ≥0.30–1.8) for tofacitinib 5 mg BID and MTX was associated with a low probability (≤10%) of achieving LDA at Mo 6. For tofacitinib 5 mg BID, failure to achieve lower thresholds of DAS28-4(ESR) improvement (range ≥0.3 to 0.9) at Mo 1 was associated with lower probability of LDA at Mo 6 than higher DAS28-4(ESR) thresholds (≥1.2–1.8) (Table).
Conclusion: Failure to achieve DAS28-4(ESR) improvement ≥1.2 from baseline at Mo 3 was associated with a low probability of achieving LDA at Mo 6 (≤6.8%) for tofacitinib 5 mg BID and MTX. For the minority of MTX-naïve RA pts treated with tofacitinib 5 mg BID or MTX who failed to achieve a minimal clinical response (DAS28-4[ESR] improvement ≥1.2) by Mo 3, another treatment option can be considered at Mo 3, as there is a low probability of reaching LDA at Mo 6. References: 1. Smolen J et al. Ann Rheum Dis 2016;75(1):3-15. 2. Lee EB et al. N Engl J Med 2014;370:2377-2386.
To cite this abstract in AMA style:
Keystone E, van Vollenhoven RF, Wilkinson B, Fallon L, Hwang LJ, Chapman D, DeMasi R, Lee EB. Lack of Early Change in Disease Activity Score Predicts the Likelihood of Achieving Low Disease Activity at Month 6: Tofacitinib Monotherapy Versus Methotrexate in Methotrexate-Naive Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/lack-of-early-change-in-disease-activity-score-predicts-the-likelihood-of-achieving-low-disease-activity-at-month-6-tofacitinib-monotherapy-versus-methotrexate-in-methotrexate-naive-patients-with-rhe/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/lack-of-early-change-in-disease-activity-score-predicts-the-likelihood-of-achieving-low-disease-activity-at-month-6-tofacitinib-monotherapy-versus-methotrexate-in-methotrexate-naive-patients-with-rhe/