Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Data on disease progression and joint involvement patterns in early oligoarticular (oligo) PsA are sparse. In the FOREMOST study (NCT03747939), apremilast (APR) decreased the number of involved joints vs placebo (PBO) at Week (Wk) 16.1 We assess patterns of involved joints at baseline and effect of APR on joint groups through Wk 48.
Methods: FOREMOST enrolled 308 patients (pts) with early oligo PsA ( >1–≤4 swollen, >1–≤4 tender joints; 66–68 joints assessed). Pts were randomized 2:1 to APR (n=203) or PBO (n=105) for 24 wks (early escape at Wk 16), after which all pts received APR through Wk 48. Overall, 291 pts received ≥1 dose of APR (as randomized, n=203; switched from PBO, n=88). Concomitant NSAIDs and/or synthetic DMARDs were permitted at a stable dose for ≥2 wks or ≥3 months before baseline, respectively, and maintained through Wk 24. For each joint group (Table 1), percentage of pts (pt incidence) with ≥1 of the following was calculated at baseline, Wks 16 and 48: swollen and tender, swollen and/or tender, swollen only, and tender only. Baseline and Wk 16 data were analyzed for all randomized pts with the last observation carried forward for missing data; Wk 48 data were analyzed as observed for pts who received ≥1 dose of APR.
Results: Pts had low baseline joint involvement: mean swollen and tender joint count, 2.6 and 3.2, respectively;1 baseline pt incidence of “swollen and tender” and “swollen and/or tender” joints was similar and higher than “swollen only” and “tender only” (Fig 1A). Based on “swollen and tender” joints, the most commonly involved joint groups at baseline included large and small joints: finger PIP (39.0% pts with ≥1 involved joint) and MCP (33.1%), followed by knee (19.8%), and ankle and tarsus/midfoot (18.8%) (Fig 1A). Baseline patterns of swollen and tender joints were similar for APR and PBO (Fig 1B). Greater improvements in swollen and tender finger PIP, MCP, knee and ankle/tarsus/midfoot joints were observed from baseline to Wk 16 for APR vs PBO: finger PIP, –23.6% vs –9.5% pt incidence; MCP, –21.7% vs –13.3%; knee –16.3% vs +2.9%; and ankle/tarsus/midfoot, –12.3% vs –8.6% (Fig. 1B, C). Across joint groups, risk of ≥1 swollen and tender joint at Wk 16 was lower for APR vs PBO (Fig. 1D), with significant differences observed for the most commonly involved joints: odds ratio (APR vs PBO) (95% CI) for finger PIP, 0.55 (0.31, 0.98); MCP, 0.51 (0.27, 0.97); knee, 0.33 (0.16, 0.71); ankle/tarsus/midfoot, 0.41 (0.17, 0.94) (Fig. 1D). Improvements in joint involvement observed with APR at Wk 16 were maintained in pts continuing or switching to APR through Wk 48.
Conclusion: Our data indicate a mix of large and small joints involved in early oligo PsA, with hand and finger most common. We observed similar joint patterns as reported at PsA onset in real-world cohorts,2,3 underscoring the value of FOREMOST in understanding early oligo PsA. Compared with PBO, APR significantly reduced joint involvement across large and small joints. Our findings can help orient physicians treating early oligo PsA.References: 1 Gossec. Ann Rheum Dis. 2024;83(11):1480-14882 Zabotti. RMD Open. 2024;10(2):e004314 3 Gladman. J Rheumatol. 2021;48(12):1824-1829
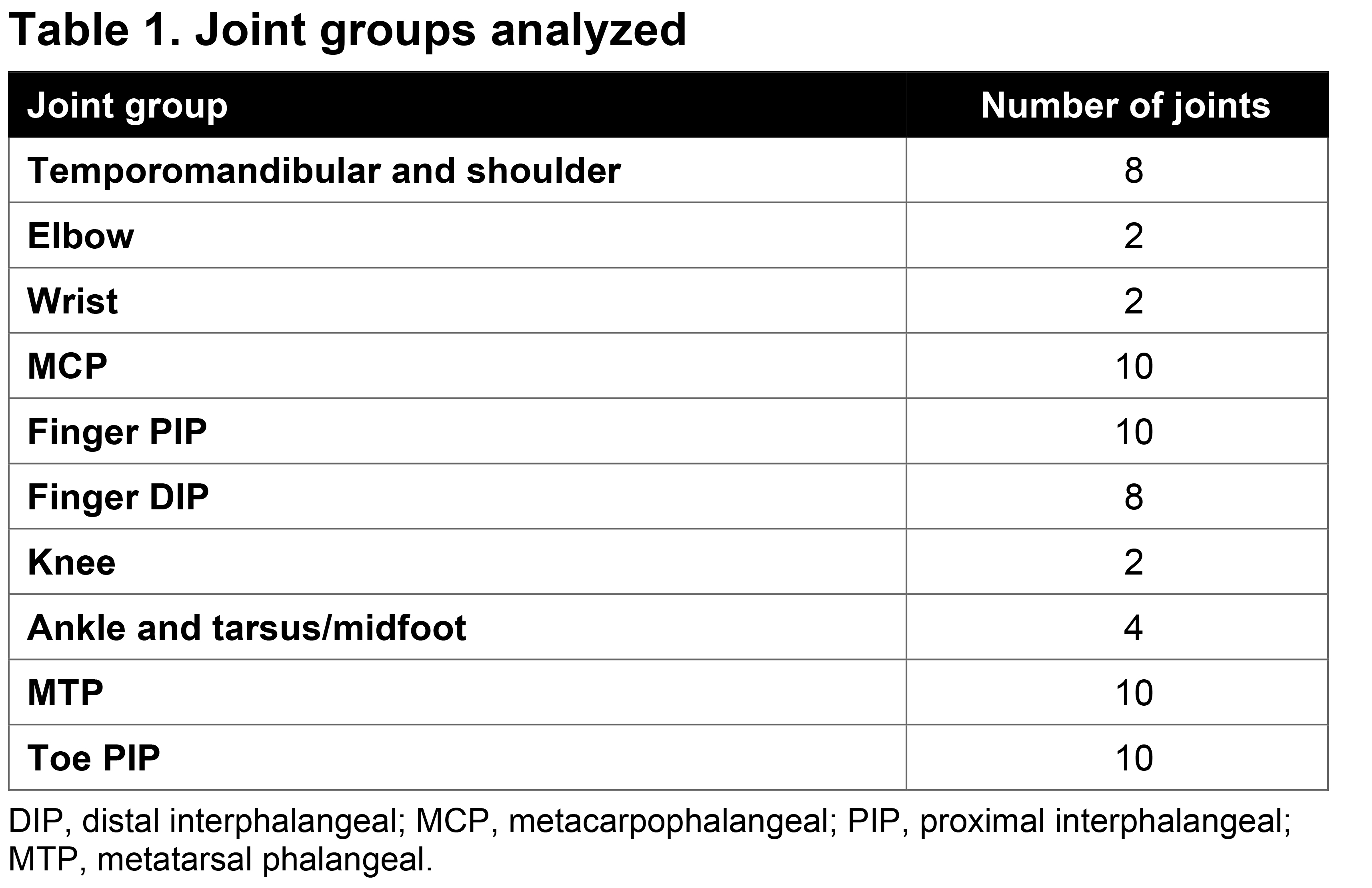 Table 1. Joint groups analyzed
Table 1. Joint groups analyzed
.jpg) Figure 1. Joint involvement in the FOREMOST study of early oligoarticular PsA
Figure 1. Joint involvement in the FOREMOST study of early oligoarticular PsA
To cite this abstract in AMA style:
Zabotti A, Merola J, Mrowietz U, Mease P, Gossec L, kishimoto m, Brunori M, Teng L, Gladman D, Coates L. Joint Groups Involved in Early Oligoarticular Psoriatic Arthritis and the Impact of Apremilast Treatment: Data From FOREMOST [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/joint-groups-involved-in-early-oligoarticular-psoriatic-arthritis-and-the-impact-of-apremilast-treatment-data-from-foremost/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/joint-groups-involved-in-early-oligoarticular-psoriatic-arthritis-and-the-impact-of-apremilast-treatment-data-from-foremost/
