Session Information
Date: Tuesday, November 14, 2023
Title: (2387–2424) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with giant cell arteritis (GCA) can relapse despite glucocorticoids, methotrexate and tocilizumab treatment. The JAK/STAT signalling pathway is involved in the pathogenesis of GCA, and JAK inhibitors (JAKi) are a potential treatment alternative. Baricitinib showed positive results in a small uncontrolled study (1).
The objective of this study is to evaluate the effectiveness of JAKi in GCA.
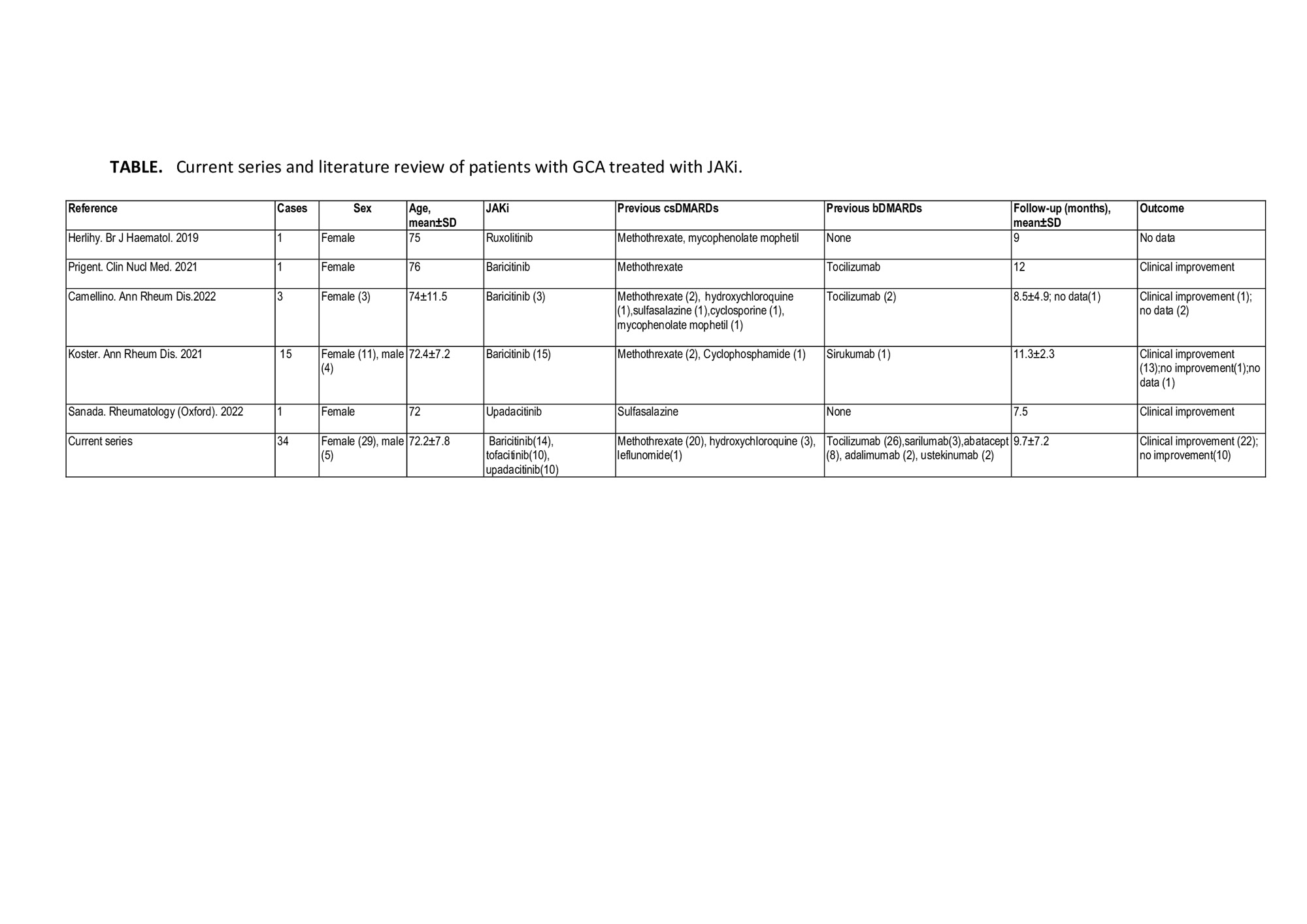
Methods: Real-world, retrospective clinical practice study of patients with GCA treated with JAKi. Outcomes assessed included disease relapse and safety. A literature search for other JAKi-treated GCA cases was conducted in PubMed, Embase and the Cochrane library from inception to 04/30/2023. We compared results of the previous baricitinib study (1) and the baricitinib recipients in our series.
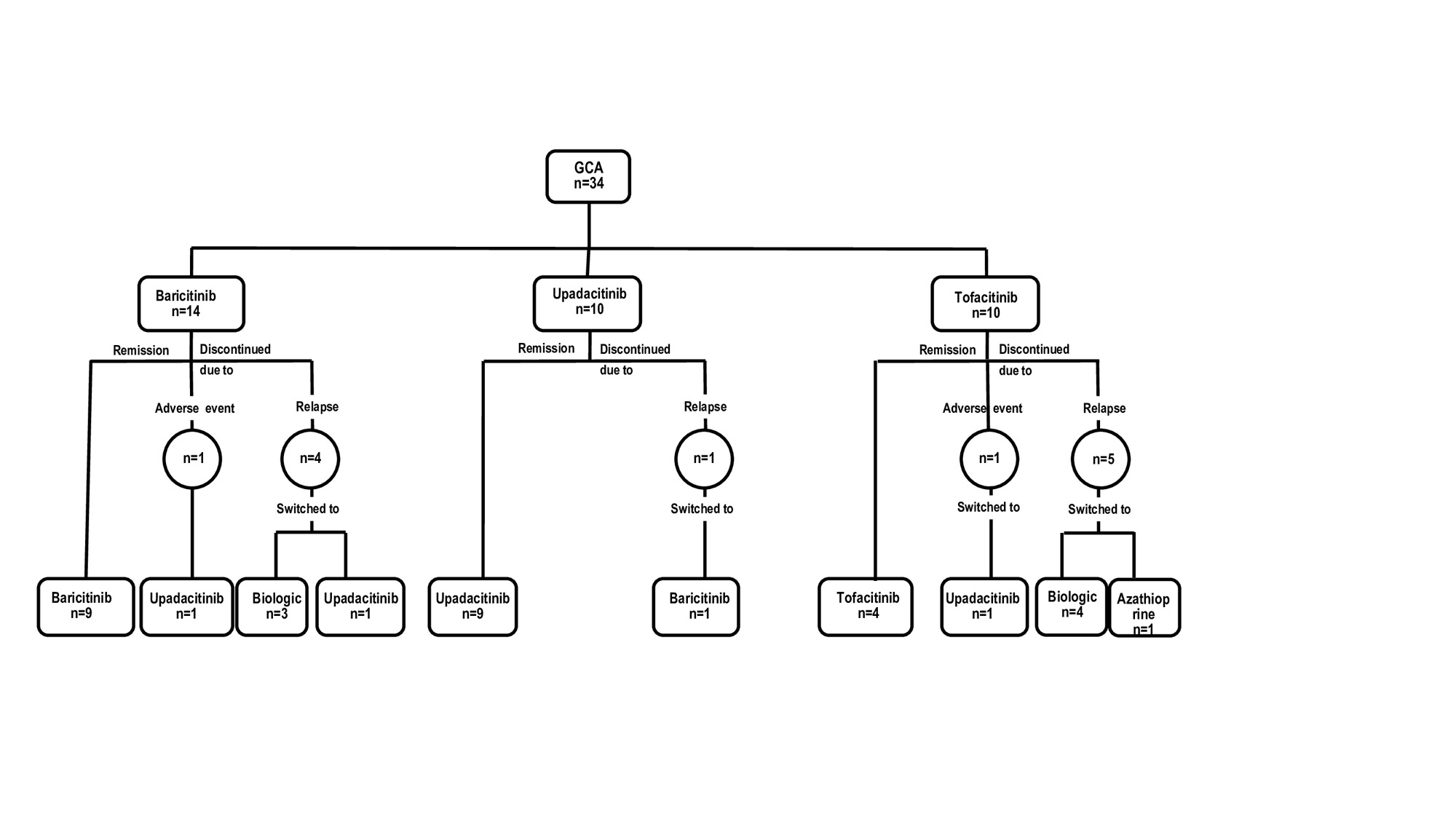
Results: We present 34 patients (29 females[85%], mean age, 72.2 years, relapsing disease 34 [100%]) that received JAKi . The initial JAKi was baricitinib (n=14), tofacitinib (n=10) and upadacitinib (n=10) (Table and Figure). After a median [IQR] follow-up of 9.5 [4.2-12.7] months, 22 (64.7%) achieved and maintained remission, and 12 (35.3%) patients discontinued the initial JAKi due to relapse (n=10, 29.4%) or severe adverse events (SAEs) (n=2, 5.9%) including liver dysfunction and dyspnea/palpitations. The 12 patients failing the initial JAKi were switched to an alternative [JAKi (n=4), biologic therapy (n=7) and azathioprine (n=1)]. The literature review identified another 21 GCA patients (17 females, mean age 74.2 years) treated with JAKi, mostly with baricitinib (n=18). Most of these patients benefited from JAKi therapy (Table). Patients in our series receiving baricitinib had longer disease duration (median [IQR]31 [12-51] vs 9 [7-21] months; p=0.001) and had received biologics (71% vs 6.7%; p< 0.001) more frequently than those in the previous baricitinib study (1). Remaining baseline features were similar.
Conclusion: This real-world analysis suggest that JAKi could be effective in GCA, including patients failing other immunosuppressive therapies. The results of an ongoing phase 3 randomized controlled trial are awaited to confirm or rule out this observation.
References:
- Koster MJ, et al. Ann Rheum Dis. 2022
To cite this abstract in AMA style:
López F, Loricera J, Tofade T, Prieto-Peña D, Romero Yuste S, De Miguel E, Riveros-Frutos A, Ferraz Amaro I, Castañeda S, Labrador-Sánchez E, Maiz O, Becerra-Fernández E, Narvaez J, Galindez-Agirregoikoa E, González I, Urruticoechea A, Unizony S, Blanco R. Janus Kinase Inhibitors in Giant Cell Arteritis in Clinical Practice. Real-World Clinical Practice Study and Literature Review [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/janus-kinase-inhibitors-in-giant-cell-arteritis-in-clinical-practice-real-world-clinical-practice-study-and-literature-review/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/janus-kinase-inhibitors-in-giant-cell-arteritis-in-clinical-practice-real-world-clinical-practice-study-and-literature-review/