Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Nail psoriasis (PsO) is a strong predictor for the development of psoriatic arthritis (PsA) and has been reported in 63–83% of patients with PsA1. Psoriatic nails are linked to arthritis in the adjacent distal interphalangeal joint (DIP) of fingers or interphalangeal joint of thumbs2, and both can lead to severe functional impairment. In the SPIRIT-H2H study of adults with PsA, 354 of 566 participants had nail PsO and adjacent joint disease in at least one digit at baseline3-4. At the group level, SPIRIT-H2H patients treated with ixekizumab (IXE) achieved significantly greater improvements in nail PsO compared to those treated with adalimumab (ADA)5. This analysis aimed to assess the treatment effects of IXE and ADA at the individual digit level among patients with PsA, nail PsO, and adjacent joint disease.
Methods: This post hoc analysis included 354 patients from SPIRIT-H2H (NCT03151551) treated with either IXE (N=186) or ADA (N=168) who had nail PsO (Nail Psoriasis Severity Index (NAPSI) total score >0) and adjacent joint disease (tenderness and/or swelling) in at least one digit at baseline. Treatment effects were assessed for each individual finger unit displaying nail PsO and adjacent joint disease; here, finger unit defines the nail and adjacent joint of an individual digit; adjacent joint refers to the DIP of fingers or the interphalangeal joint of thumbs. Nail PsO was measured using NAPSI in the fingers only. Joint involvement was measured by tender/swollen joint count scores (TJC68/SJC66). Patients were evaluated for both nail and joint involvement at baseline and Weeks 12, 16, 24, 32, 40, and 52. Proportions of finger units with resolution of nail PsO, and proportions of finger units with resolution of adjacent joint disease were analyzed using Chi-square tests. Non-responder imputation was used to handle missing data.
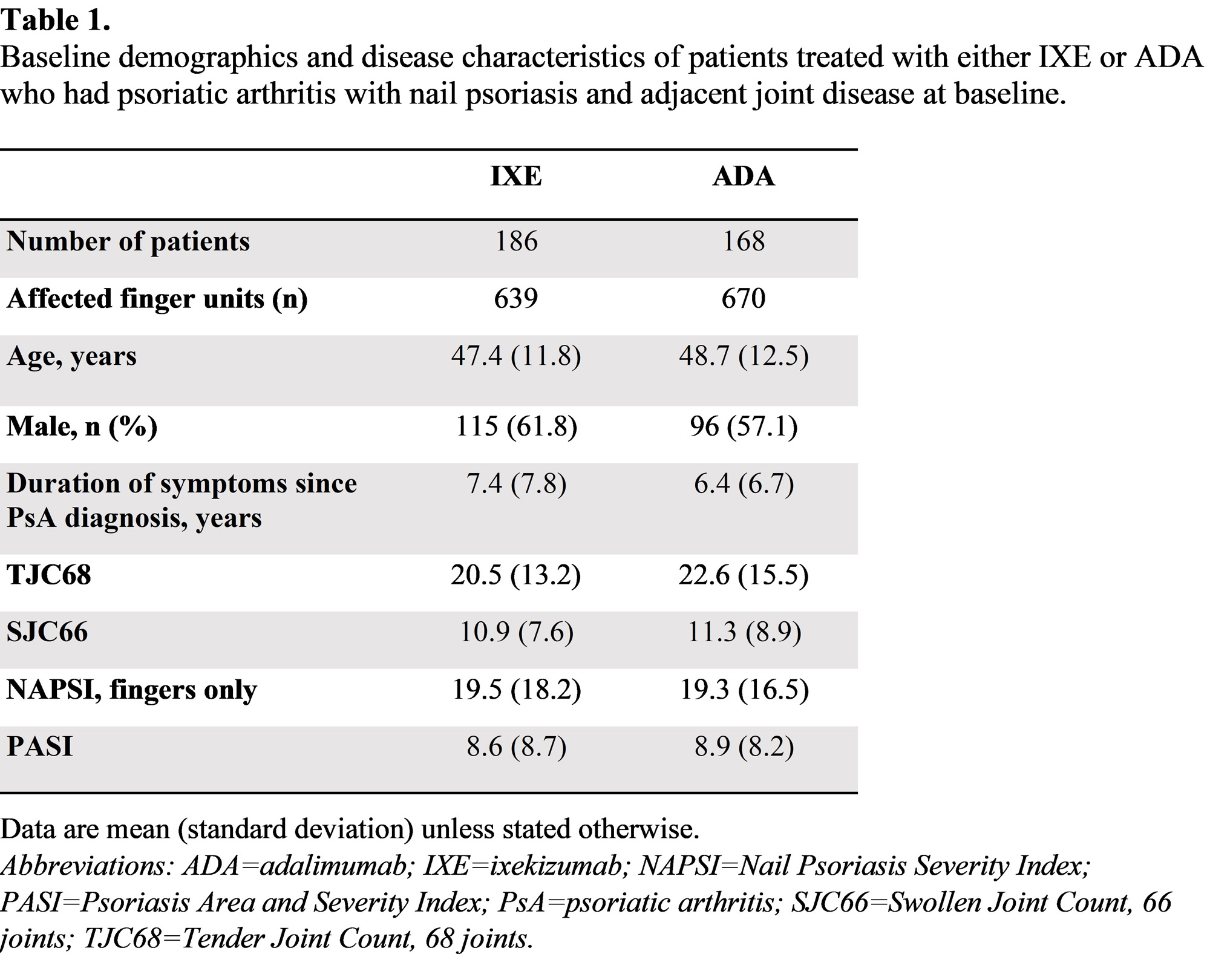
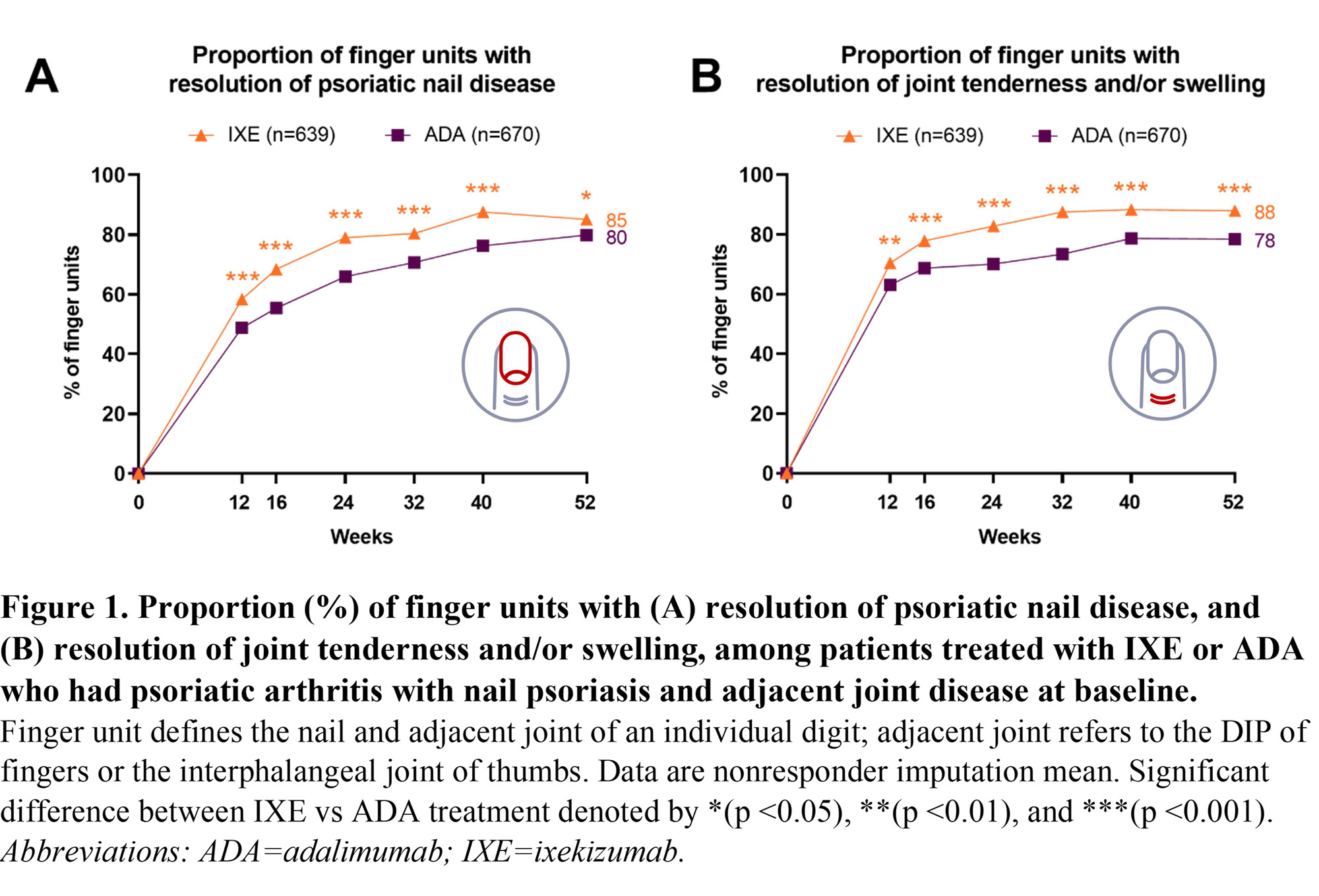
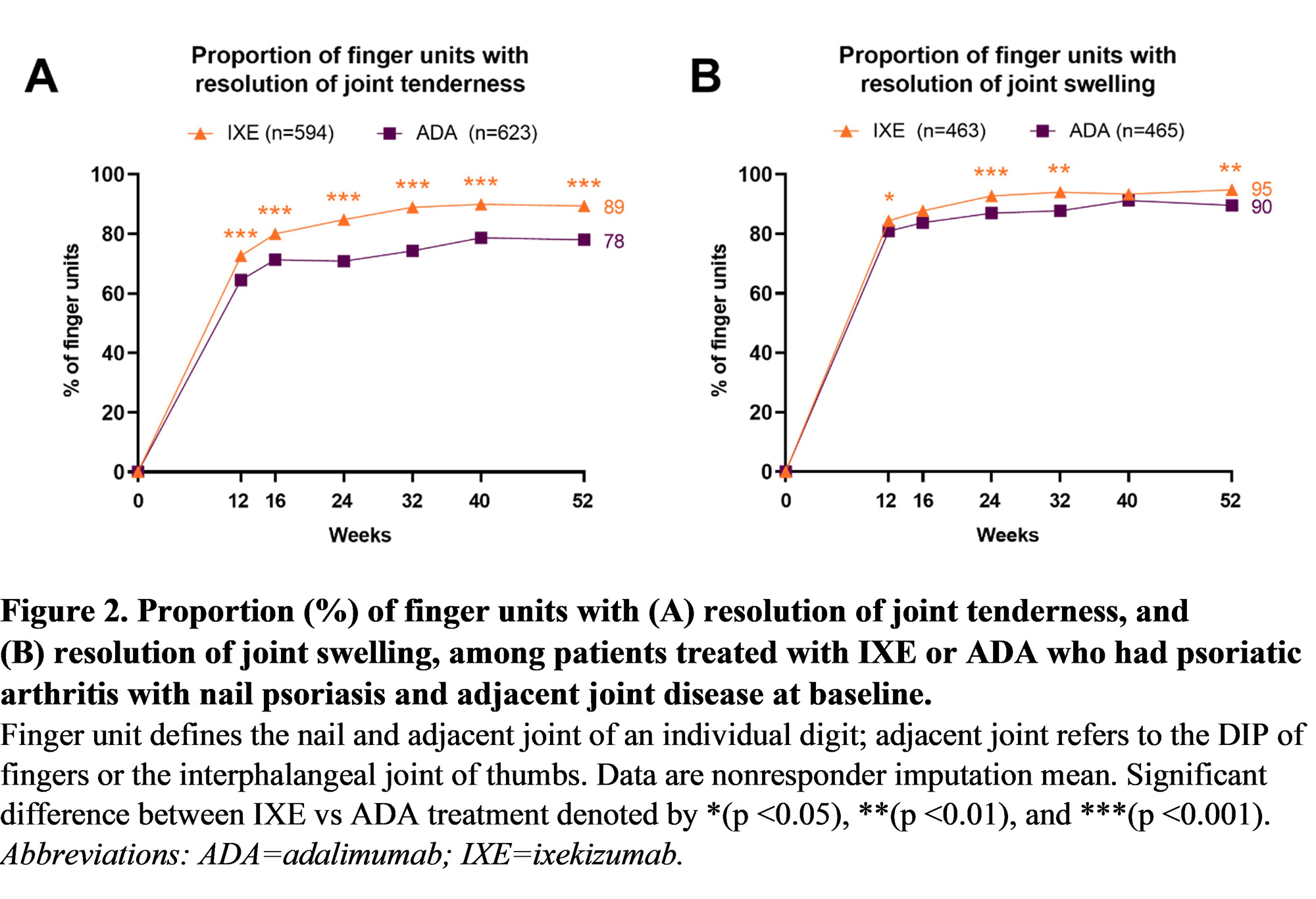
Results: There were 1309 (IXE=639, ADA=670) finger units affected by nail and adjacent joint disease at baseline (Table 1). Resolution of psoriatic nail disease (Figure 1A) and resolution of adjacent tenderness and/or swelling (Figure 1B) of the finger unit was significantly higher with IXE vs ADA at all post-baseline assessments over 52 weeks. Joint tenderness was resolved in a significantly larger proportion of IXE-treated finger units vs ADA at all post-baseline assessments over 52 weeks (Figure 2A). Joint swelling was resolved in a larger proportion of IXE-treated finger units vs ADA, and these differences reached statistical significance at all visits except Week 16 and Week 40 (Figure 2B). The tenderness and/or swelling of the finger unit resolved more rapidly than the adjacent nail disease (Figure 1).
Conclusion: IXE treatment showed a significant advantage over ADA in resolving nail PsO, joint tenderness, and joint swelling among the finger units with nail and adjacent joint disease of patients with PsA.
References
1. Elkayam O et al. Clin Rheumatol. 2000; 19(4): 301-305.
2. Lai TL et al. Clin Rheumatol. 2016; 35(8): 2031-2037
3. McGonagle D et al. British Society for Rheumatology. 2023; P169
4. McGonagle D et al. European Alliance of Associations for Rheumatology. 2023; POS1526
5. Smolen JS et al. Rheumatol Ther. 2020;7(4):1021-35
To cite this abstract in AMA style:
McGonagle D, Kavanaugh A, McInnes I, Erik L, Merola J, Strober B, Bolce R, Lisse J, Pustizzi J, Sapin C, Ritchlin C. Ixekizumab Significantly Improves Nail Disease and Adjacent Joint Tenderness and Swelling in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/ixekizumab-significantly-improves-nail-disease-and-adjacent-joint-tenderness-and-swelling-in-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-significantly-improves-nail-disease-and-adjacent-joint-tenderness-and-swelling-in-psoriatic-arthritis/