Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The Assessment of SpondyloArthritis international Society Health Index (ASAS HI) measures health, disability, and functioning in patients (pts) with spondyloarthritis (SpA)[1,2]. The purpose of this study was to assess the impact of 52 weeks of ixekizumab (IXE) treatment on overall functioning and health in 2 phase 3 trials of pts with ankylosing spondylitis (AS)/radiographic axial SpA (r-axSpA) who were either biologic (b)DMARD-naïve (COAST-V [NCT02696785]) or who previously failed or were intolerant to up to 2 TNF-inhibitors (TNF-experienced) (COAST-W [NCT02696798]).
Methods: Pts were originally randomized equally to IXE 80mg every 2 weeks (Q2W), every 4 weeks (Q4W), placebo (PBO) or (in COAST-V only) to adalimumab (ADA) 40mg Q2W. At Week16, original PBO and ADA patients were rerandomized 1:1 to either IXE regimen. All pts were treated for up to 52 weeks. Change from baseline in ASAS HI (score 0-17 with higher score indicating worse health [1,2]) up to Week 16 was analyzed using a mixed-effects model of repeated measures. Changes beyond Week 16 through Week 52 (only for pts originally randomized to IXE) were summarized with missing data imputed using modified baseline observation carried forward (mBOCF) approach. Categorical data were summarized with nonresponder imputation for missing data. For the ASAS HI, the smallest detectable change [SDC] was reported as 3.0 and patients having an ASAS HI total score ≤ 5 were defined as being in a good health state [2].
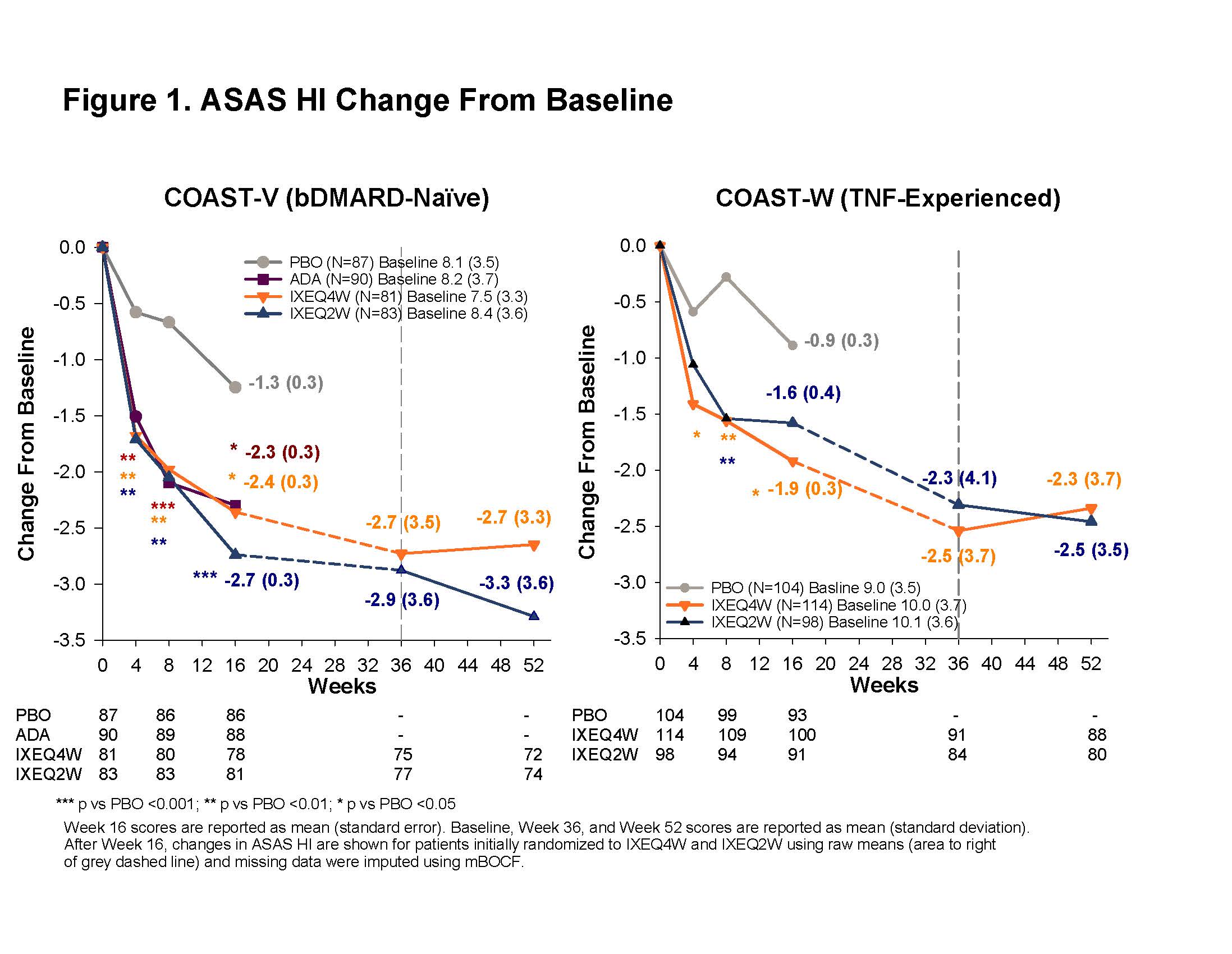
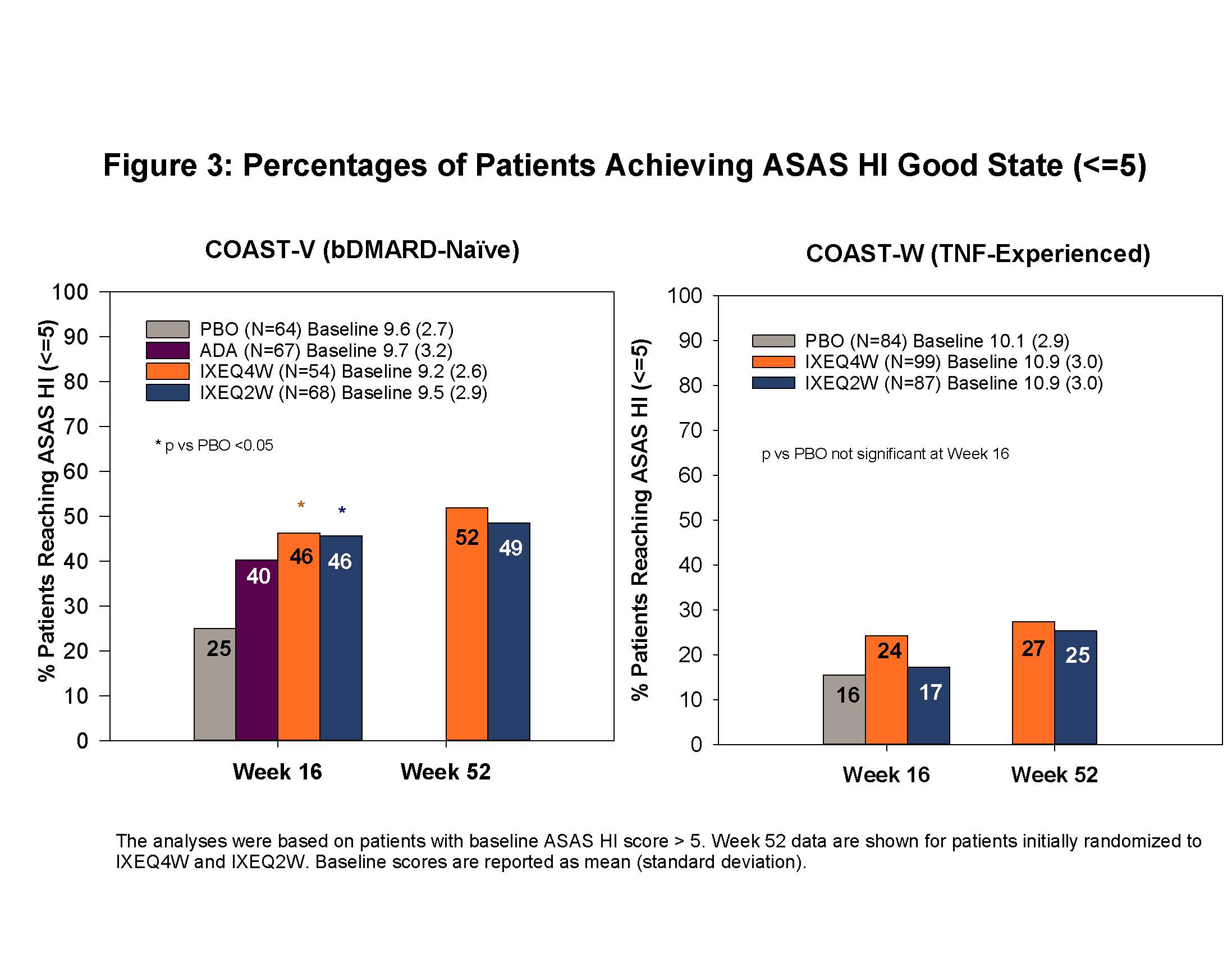
Results: At baseline, mean (standard deviation) ASAS HI scores were 8.1 (3.6) and 9.7 (3.6) in COAST-V and COAST-W, respectively. At Week 16, IXEQ4W-treated pts had improved ASAS HI scores versus PBO in both trials [3, 4]. Improvement was sustained or further increased through Week 52 (Figure 1). The proportions of pts continuously treated with IXE and having reached SDC (Figure 2) and achieving ASAS HI good state (Figure 3) were maintained at Week 52. Among pts originally randomized to PBO but switched to IXE at Week16, the percentages of pts with improvement above SDC or achieving an ASAS HI good state through 52 weeks were 45% and 36% in bDMARD-naive, and 41% and 31% in TNF-experienced pts, respectively.
Conclusion: Continued treatment with IXE through 52 weeks led to sustained improvement in ASAS HI as measured by change from baseline, and proportions of pts achieving a change above SDC or good ASAS HI state in both in bDMARD-naïve and in TNF-experienced pts.
References
- Kiltz, et al. Ann Rheum Dis. 2015;74:830-835.
- Kiltz , et al. Ann Rheum Dis. 2018;77:1311-1317.
- van der Heijde, et al. Lancet 2018;392:2441-2451.
- Deodhar, et al. Arthritis Rheumatol. 2019;71-599-611.
To cite this abstract in AMA style:
Kiltz U, van der Heijde D, Boonen A, Gensler L, Hunter T, Zhao F, Zhu B, Bolce R, Carlier H, Braun J. Ixekizumab Improves Self-reported Overall Functioning and Health as Measured by the Assessment of SpondyloArthritis International Society Health Index in Patients with Active Radiographic Axial Spondyloarthritis: 52-Week Results of Two Phase 3 Randomized Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/ixekizumab-improves-self-reported-overall-functioning-and-health-as-measured-by-the-assessment-of-spondyloarthritis-international-society-health-index-in-patients-with-active-radiographic-axial-spondy/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-improves-self-reported-overall-functioning-and-health-as-measured-by-the-assessment-of-spondyloarthritis-international-society-health-index-in-patients-with-active-radiographic-axial-spondy/