Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Axial spondyloarthritis (axSpA) is an inflammatory condition affecting sacroiliac joints and axial skeleton, characterized by fatigue, spinal pain, and sleep disturbances that negatively affect patient health-related quality of life.1 Ixekizumab (IXE) has demonstrated efficacy in treatment of radiographic axial spondyloarthritis (r-axSpA) in both biologic disease-modifying antirheumatic drug (bDMARD) naïve and tumor necrosis factor inhibitor (TNFi) experienced patients.2,3 The purpose of this analysis was to evaluate the impact of IXE on fatigue, spinal pain and sleep pattern in patients with r-axSpA over 52 weeks.
Methods: COAST-V and COAST-W were Phase 3 randomized controlled trials (RCTs) conducted to evaluate the efficacy and safety of IXE in bDMARD-naïve and TNFi-experienced patients with r-axSpA, respectively. In both RCTs, patients were randomized to receive IXE 80mg once every two weeks (IXEQ2W), IXE 80 mg once every 4 weeks (IXEQ4W), active reference arm (adalimumab 40 mg [ADAQ2W]) in COAST-V or placebo (PBO) up to Week 16. Patients initially randomized to IXE continued on the same regimen up to Week 52. Patients on PBO were re-randomized to either IXEQ2W or IXEQ4W at Week 16. After a washout period of 6 weeks, patients on ADA were switched to either IXEQ2W or IXEQ4W regimens and received their first IXE dose at Week 20. Fatigue Numeric Rating Scale (NRS) and Jenkins Sleep Evaluation Questionnaire (JSEQ) data were collected at baseline, Weeks 8, 16, 36 and 52. Patient global assessments of disease activity, spinal pain and spinal pain at night were collected at baseline and each post-baseline visit to Week 52. Mean changes from baseline up to Week 16 were analyzed using mixed effects model of repeated measures (MMRM). After Week 16, changes from baseline during extended treatment period were summarized as raw means after imputing the missing data using modified Baseline Observation Carried Forward (mBOCF).
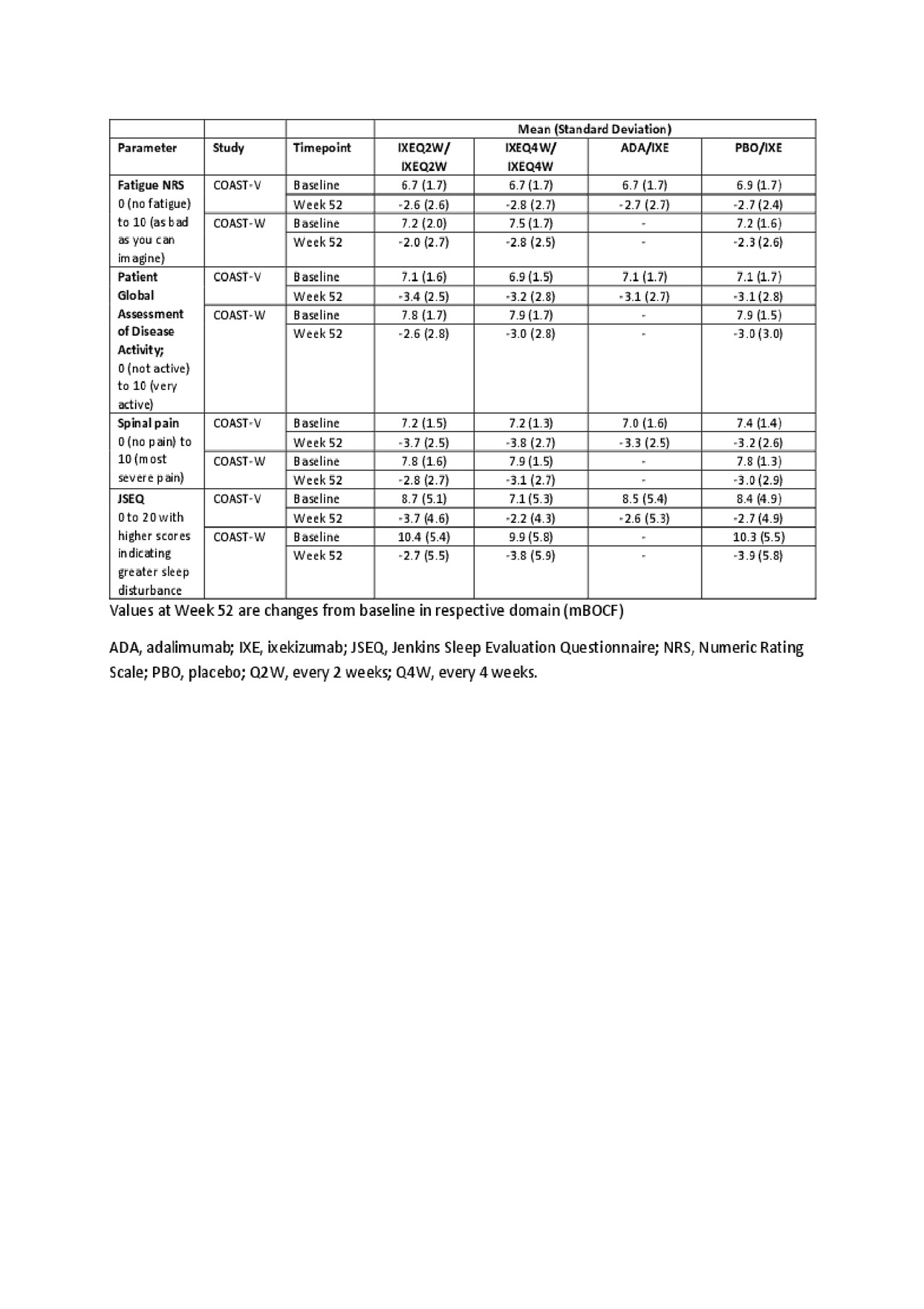
Results: At Week 52, IXEQ2W and IXEQ4W treatments continued to improve fatigue, patient global assessment of disease activity, spinal pain and sleep in bDMARD-naive and TNFi-experienced patients (Table 1). Significant reductions in fatigue and spinal pain at night were reported as early as Week 8 and Week 1 respectively, in patients treated with IXE compared with PBO. Improvements in these parameters at Week 16 with both IXE arms were maintained up to Week 52 (Figure 1).
Conclusion: Fatigue, spinal pain, and sleep improved with IXE treatment over 52 weeks in both biologic-naïve and TNFi-experienced patients with r-axSpA.
References
- Strand V, et al. J Clin Rheumatol. 2017;23(7):383–391.
- van der Heijde D, et al. Lancet. 2018;392(10163):2441-2451.
- Deodhar A, et al. Arthritis Rheumatol. 2019;71(4):599-611.
To cite this abstract in AMA style:
Deodhar A, Mease P, Rahman P, Navarro-Compán V, Strand V, Hunter T, Sandoval D, Lisse J, Zhao F, Marzo-Ortega H. Ixekizumab Improves Fatigue, Pain, and Sleep up to 52 Weeks in Patients with Radiographic Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/ixekizumab-improves-fatigue-pain-and-sleep-up-to-52-weeks-in-patients-with-radiographic-axial-spondyloarthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ixekizumab-improves-fatigue-pain-and-sleep-up-to-52-weeks-in-patients-with-radiographic-axial-spondyloarthritis/