Session Information
Date: Monday, October 22, 2018
Title: Patient Outcomes, Preferences, and Attitudes Poster I: Patient-Reported Outcomes
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Pain and fatigue are common symptoms for patients with rheumatoid arthritis (RA).
JAK inhibitors (JAKi) already proved similar efficacy on disease activity as bDMARD (anti-TNF, anti-IL6, abatacept and rituximab). Moreover, some trials suggest that JAKi may have a supplementary interest on the patient’s reported outcomes (PRO). Based on a systematic review of the literature, our goal was to determine the impact of each treatment on the pain and fatigue.
Methods: We screened the literature for clinical trial comparing biological DMARDs or one of the JAKi to placebo for pain and/or fatigue, using Pubmed, Cochrane Library, Embase. For JAKi, we restricted our investigation to baricitinib and tofacitinib, that are registered by the FDA and the EMA in RA treatment. Among 1488 articles initially identified, 33 randomized controlled trials evaluated pain VAS and 17 investigated fatigue using the FACIT-F score were selected. Data was extracted independently by two authors. For each study, we calculated the change of VAS pain and FACIT between baseline and study endpoint for the DMARDs and the placebo. Meta-analyses were performed to estimate pooled mean difference (MD) with their 95% confidence interval using the inverse variance approach. Heterogeneity was assessed (Cochran’s Q-test and I2).
Results: The result of pain improvement with all bDMARDs versus Placebo is MD: -12,94 (IC95% (-15,32; -10,57); I2=79%). In subgroup analysis, for antiTNFs MD: -13.97 (IC95% (-15,92; -12,02); I2=30%), for anti-IL6 MD:-8,81 (IC95% (-14,54;-3,08);I2=86%), for Abatacept MD: -12,27 (IC95% (-18,90; -5,63); I2=83%) and for Rituximab MD: -17,87 (IC95% (-23,85; -11,9); I2=82%). For the JAKi, improvement of pain is MD: -13,81 (IC95% (-16,46 ; -11,16); I2=70%) (figure 1).
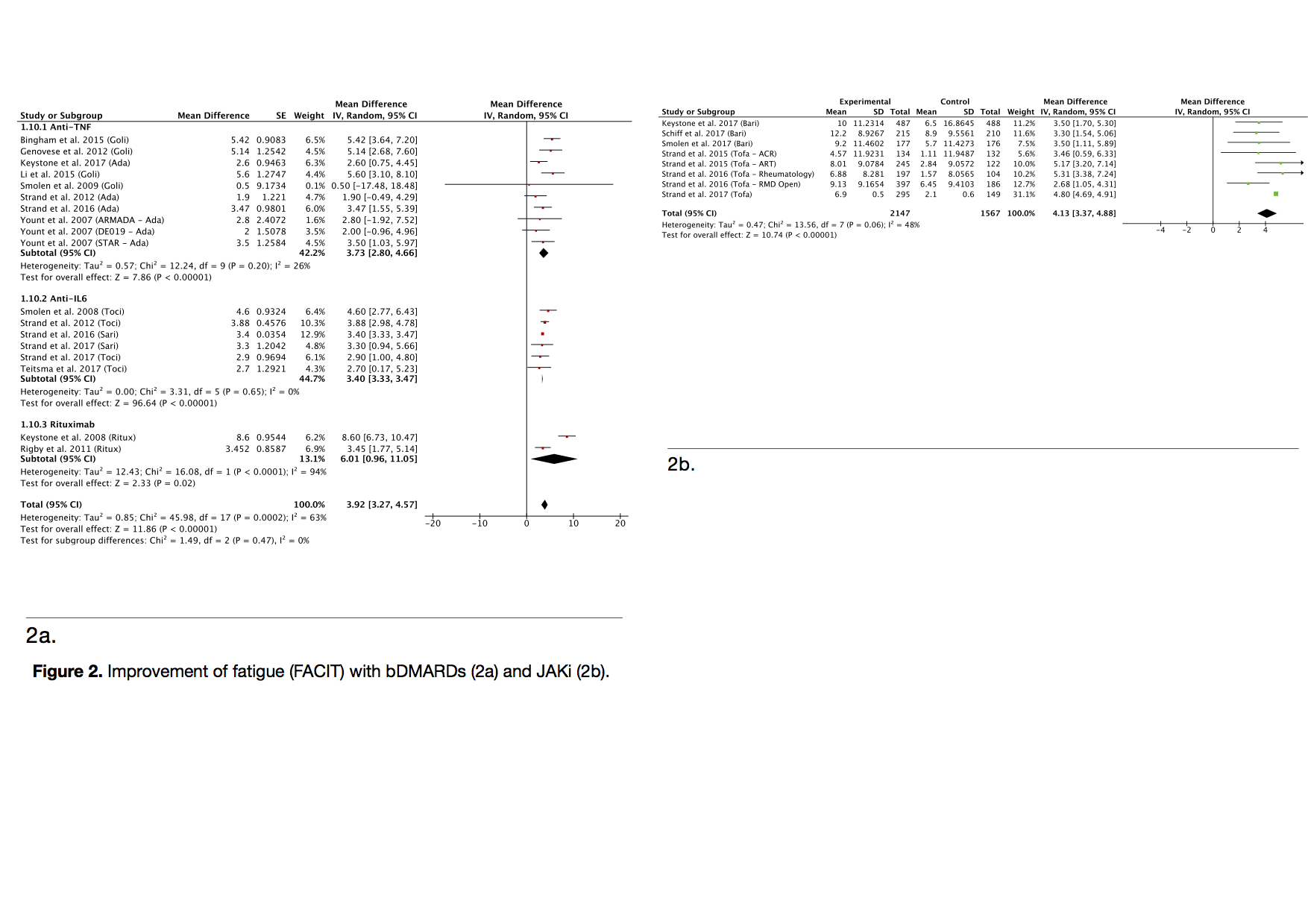
Fatigue improvement with bDMARDs versus Placebo is MD: 3,92 (IC95% (3,27; 4,57); I2=63%). In subgroup analysis, for antiTNFs MD: 3,73 (IC95% (2.80; 4,66); I2=26%), for anti-IL6 MD: 3,4 (IC95% (3,33; 3,47);I2=0%), and for Rituximab MD: 6,01 (IC95% (0,96; 11,05) I2=94%). For the JAKi, MD: 4,13 (IC95% (3,37; 4,88); I2=48%) (figure 2).
Conclusion: Our results indicate that the impact of JAKi on pain and fatigue is not significantly different than biologic DMARDs, at least during the short and middle term corresponding to the duration of clinical trials.
To cite this abstract in AMA style:
Odriozola I, Coste CS, Barnetche T, Richez C, Bannwarth B, Schaeverbeke T. Is There a Specific Effect of Jak-Inhibitors on Pain and Fatigue in Rheumatoid Arthritis? [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/is-there-a-specific-effect-of-jak-inhibitors-on-pain-and-fatigue-in-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-there-a-specific-effect-of-jak-inhibitors-on-pain-and-fatigue-in-rheumatoid-arthritis/