Session Information
Session Type: ARHP Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is used for the long-term treatment of systemic lupus erythematosus (SLE) patients. HCQ is generally well-tolerated with wide-ranging benefits, including reduced disease activity and improved survival; a critical adverse effect is vision-threatening retinopathy. Historically, the risk was considered to be very low. However, a recent retrospective case-control study (N=2,361 taking HCQ for ≥5 years) reported the prevalence estimate of HCQ retinopathy to be 7.5%, which is >3 times higher than that of previous studies (Melles R, et al. JAMA Ophthalmol.; 2017). This study served as the key basis for the 2016 revised American Academy of Ophthalmology (AAO) recommendations for HCQ retinopathy screening. However, this study did not take into account the competing risk of death (e.g., a 2-fold increased mortality risk in SLE) and thus, the reported retinopathy risk could be overestimated. We conducted a simulation study to estimate potential impact of competing risk of death on risk of retinopathy among patients with SLE.
Methods: In the simulation studies we assumed that (1) the hazard rates of toxic retinopathy for three different doses of HCQ (i.e., >5mg/kg), 4-5mg/kg and <4mg/kg) change over time according to a Weibull distribution; (2) the survival rate in the general population from 55 to 75 years old was based on 2013 Life Table in US; (3) mortality rate in SLE patients is 2-fold higher than that in the general population; and (4) the risk of retinopathy was 70%, 40%, and 15% for 25 years, respectively, based on the previous study. We estimated the risk of retinopathy and depicted survival curves using K-M curve with and without accounting for competing risk of death. We simulated 6000 independent observations based on 1000 replications.
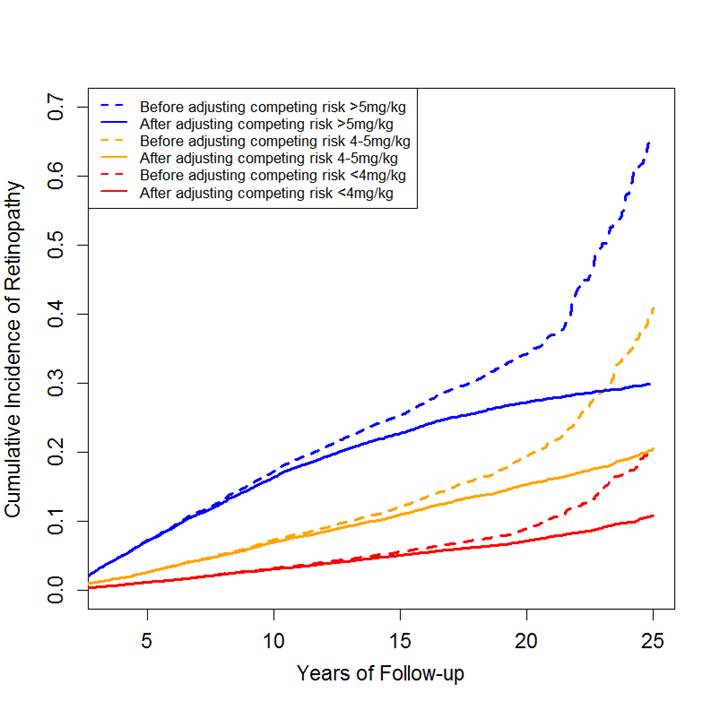
Results: As shown in Figure 1, the shape and magnitude of risk of retinopathy for each HCQ dose category were similar to the findings of the previous study. After accounting for competing risk of death, the risk of toxic retinopathy was 29.9% (95% CI: 28.8% to 30.1%), 20.4% (95% CI: 19.4% to 21.5%), 10.7% (95% CI: 9.9% to 11.6%), respectively, for each dose over 25 years. The risk of retinopathy from HCQ without accounting for competing risk death may overestimate the true risk of retinopathy for each category of HCQ by 40.2%, 96.0% and 134.1% over 25 years of use, respectively.
Conclusion: Although high dose and long-term HCQ use increase the risk of toxic retinopathy, the reported risk of retinopathy from the recent study without accounting for competing risk of deaths in SLE patients is likely to be overestimated particularly in the long-term follow-up. Prospective studies are needed to accurately estimate the risk of retinopathy attributed to HCQ.
To cite this abstract in AMA style:
Lu N, Choi HK, Jorge A, Zhang Y. Is Risk of Retinopathy Among Hydroxychloroquine Users of SLE Patients Accurate? a Simulation Study Accounting for Competing Risk of Death [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/is-risk-of-retinopathy-among-hydroxychloroquine-users-of-sle-patients-accurate-a-simulation-study-accounting-for-competing-risk-of-death/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-risk-of-retinopathy-among-hydroxychloroquine-users-of-sle-patients-accurate-a-simulation-study-accounting-for-competing-risk-of-death/