Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In Israel, patients treated with adalimumab (ADA) can enroll in a patient support program (PSP) provided by AbbVie, which includes a home visit by a registered nurse, telephone calls, and outreach by mail. Clalit Health Services (CHS), a payer/provider system with over four million members, maintains a complete electronic medical record database, which can be used to track the effect of PSP on outcomes. The objective of this study was to examine whether PSP participation is associated with a longer persistence and/or a greater adherence to ADA.
Methods: This is a retrospective cohort study of new ADA users, comparing PSP enrollees (PSP group) vs. those that did not enroll (non-PSP group) using patient-level data from the CHS database. The index date was defined as the date of the patients’ first purchase of ADA occurring between 01/08/2012 and 31/12/2014. Demographic and clinical characteristics were compared between groups at baseline. Persistence was assessed using survival analyses of time until discontinuation, defined as the date of the last purchase or prescription preceding a 90-day gap without a purchase. Adherence (medication possession ratio) among patients persistent on ADA was compared between PSP and non-PSP groups using the Mann-Whitney U test during the 0-6 months, 0-12 months, and 12-24 months following the index. Subgroup analyses were conducted by clinical indications (rheumatoid disease (RD), inflammatory bowel disease (IBD), and dermatologic disease).
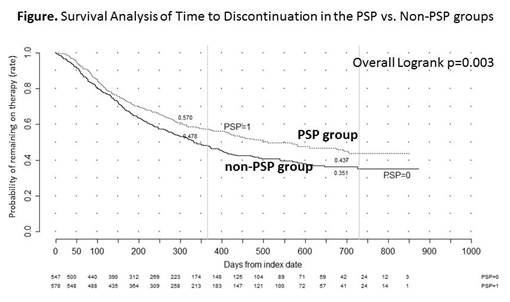
Results: There were 755 patients in the PSP group and 765 in the non-PSP group who met the inclusion criterion. Baseline characteristics were similar between cohorts. Among those with at least 6 months of follow-up post-index date, the PSP group had significantly longer mean persistence as compared to non-PSP group (319 vs. 298 days, respectively; p=0.003). Subgroup analyses yielded similar findings for the clinical indications of RD (p=0.015) and IBD (p=0.031). Further, at 12 and 24 months, the PSP group was more likely to be persistent on medication as compared to the non-PSP group: 57.0% vs. 47.8% (p=0.002) and 43.7% vs. 35.1% (p=0.003), respectively (Figure). The mean adherence rates among those with varying lengths of persistence were all greater than 80%. The 6-month adherence rate among those with at least 6 months persistence was significantly greater for the PSP group compared to the non-PSP group (p=0.024). Subgroup analyses yielded similar finding in the clinical indication of RD (p=0.046).
Conclusion: A patient support program provided by Abbvie was associated with a longer persistence among new users of ADA. It was also associated with a higher adherence rate within the first 6 months.
To cite this abstract in AMA style:
Srulovici E, Garg V, Ghilai A, Feldman B, Hoshen M, Balicer R, Skup M, Leventer-Roberts M. Is Patient Support Program (PSP) Participation Associated with Longer Persistence and Greater Adherence Among New Users of Adalimumab? [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/is-patient-support-program-psp-participation-associated-with-longer-persistence-and-greater-adherence-among-new-users-of-adalimumab/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-patient-support-program-psp-participation-associated-with-longer-persistence-and-greater-adherence-among-new-users-of-adalimumab/