Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
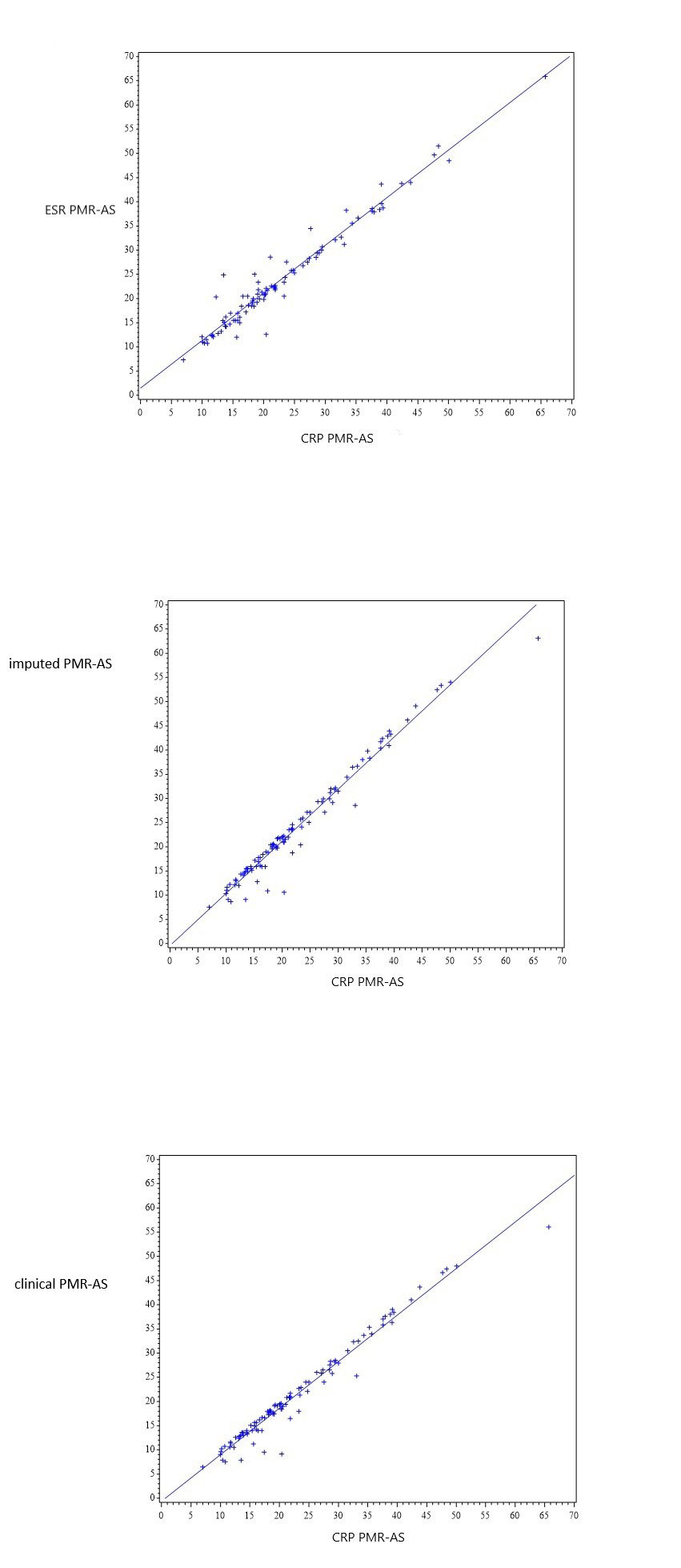
Background/Purpose: Today, the only disease activity score developed specifically for polymylagia rheumatica is the PMR-AS. It is a composite index built as an algebraic sum of five different item of interest in PMR (morning stiffness, elevation of the upper limbs, physician’s global assessment, patient’s pain assessment and CRP). However, it can have some limitations because C-reactive protein (CRP) values are not always obtained during follow-up, and treatments such as IL-6 antagonists or corticosteroid can normalize CRP level. Derived PMR-AS have been described to avoid such bias: ESR PMR-AS, Clinical PMR-AS and imputed PMR-AS but the concordance and the correlation between these scores are unknown. Our objective is to measure the correlation and concordance of the PMR-AS (also called CRP PMR-AS) and the ESR PMR-AS, Clinical PMR-AS and imputed PMR-AS.
Methods: We used patients data from the SEMAPHORE trial (Safety and Efficacy of tocilizumab versus Placebo in Polymyalgia rHeumatica With glucocORticoid dEpendence, ), a superiority randomized double-blind parallel placebo-controlled trial in patients with cortico-dependent PMR, investigated whether tocilizumab resulted in higher rates of low disease activity combined with a significant glucocorticoid-sparing effect compared to a placebo, after 24 weeks. Primary end point was based on PMR-AS and secondary end points evaluated ESR PMR-AS (CRP is replaced by ESR in the sum), Clinical PMR-AS (CRP is not included) and imputed PMR-AS [CRP-imp PMR-AS = 1.12(clinical PMA-AS)+0.26]. Using kappa coefficient, scatter plots and Bland Altman graphics, we evaluated the concordance and correlation between each PMR-AS on the whole population and by treatment group (placebo versus Tocilizumab) for different cut offs (1.5; 7; 10; 17) at each visit.
Results: 100 patients received at least one dose of tocilizumab (49 patients) or placebo (51 patients) and were included in the analyses from inclusion to week 24. We found an excellent correlation between CRP PMR-AS and the other activity scores (figure 1) in the whole population and in each treatment group at inclusion and W24. Bland Altman graphics using CRP PMR-AS as a reference showed that difference are low irrespective to the value. ICC were all higher than 0.9 (table 1). Kappa coefficients for the different cut- offs were also high between each PMR-AS at week 24.
Table 1: ICC between different PMR-AS for the whole population
| ICC (all patients all visits) | ICC (95% CI) |
| CRP PMR-AS and ESR PMR-AS | 0.991 (0.990-0.992) |
| CRP PMR-AS and Clin PMR-AS | 0.991 (0.990-0.992) |
| CRP PMR-AS and Imp PMR-AS | 0.988 (0.987-0.989) |
| Kappa- W24 (cut off 10) | Kappa |
| CRP PMR-AS and ESR PMR-AS | 0.9584 (0.9013-1) |
| CRP PMR-AS and Clin PMR-AS | 0.9143 (0.8324-0.9963) |
| CRP PMR-AS and Imp PMR-AS | 0.8549 (0.7515-0.9583) |
Conclusion:
The correlation between different PMR-AS scores using or not CRP are excellent reflecting the low weight of CRP in the reference composite index. In clinical trial using drugs that have a high impact on CRP, CRP-PMR-AS score or others can be used.
To cite this abstract in AMA style:
D'agostino J, Devauchelle V, CARVAJAL ALEGRIA G, Dernis E, Richez C, Truchetet M, Wendling D, TOUSSIROT E, Perdriger a, gottenberg j, FELTEN R, Fautrel B, chiche l, HILLIQUIN P, Le Henaff C, Dervieux B, Direz G, Chary-Valckenaere I, CORNEC D, Guellec D, MARHADOUR T, Souki A, Nowak E, Saraux A. Is It Possible to Evaluate Polymyalgia Rheumatica Without C-reactive Protein? Concordance and Agreement Between Different PMR Activity Scores in Polymyalgia Rheumatica [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/is-it-possible-to-evaluate-polymyalgia-rheumatica-without-c-reactive-protein-concordance-and-agreement-between-different-pmr-activity-scores-in-polymyalgia-rheumatica/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-it-possible-to-evaluate-polymyalgia-rheumatica-without-c-reactive-protein-concordance-and-agreement-between-different-pmr-activity-scores-in-polymyalgia-rheumatica/