Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Belimumab (BLM) is an IgG-1l anti-BAFF monoclonal antibody effective in patients with systemic lupus erythematosus (SLE), being used increasingly. However, there are no published data on dose optimization in responder patients.
Aim: to assess the prevalence of dose optimization in patients with SLE treated with BLM in our country, its modalities and its impact on disease activity control.
Methods: Retrospective longitudinal and multicenter study of SLE patients, treated with
BLM in Spanish rheumatology departments. Demographic and clinical features, activity (SLEDAI), treatments and outcomes (remission (DORIS -2021) and low disease activity (LLDAS) were collected at baseline (pre-optimization) (VB), at 6 (V6M) and at 12 months (V12M) post-optimization. A comparative analysis was performed pre- and post-optimization.
Results: 324 patients with SLE (98%, ACR-97 or SLICC-12 criteria) treated with BLM were included; 91% women; mean age (±DS): 42.4 (±12.9) years. Median follow-up follow-up: 3.2 years (p25-p75) (1.4-5.9). A total of 29 patients (8.9%) were optimized. Median time to optimization 2.7 (1.77-4.48) years. Mean time on optimization: 11.36 (±2.5) months. Optimization modalities: increase of the interval in 9 patients with subcutaneous BLM (from 7 days to 10-21 days of interval between doses) and in 6 patients with intravenous (ivBLM) (from a 4-week to a 5- 6-weeks). Dose reduction per administration (all ivBLM) in 16 patients (from 10mg/kg to 5-9 mg/kg).
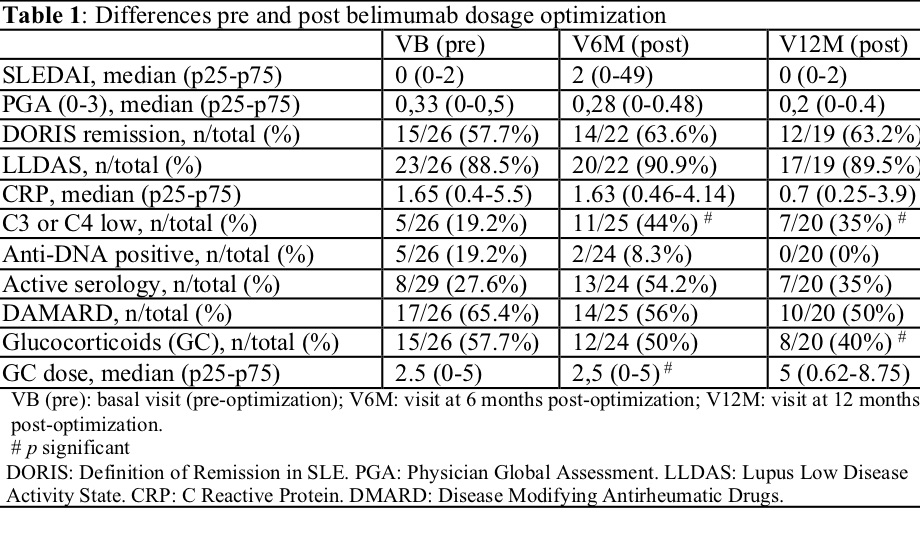
Pre-optimization status (VB): 15/26 (57.7%) in DORIS-21 remission and 23/26 (88.5%) in LLDAS. LLDAS. Only 1 patient had lupus nephritis. Detailed description of other baseline characteristics are displayed in Table 1. After optimization, 2/24 (8.3%) and 3/22(13.6%) patients lost remission-DORIS in V6M and V12M, respectively, but with no statistically significant differences with respect to VB; regarding to LLDAS, 2/23(8.7%) and 2/21(9.5%) did so in V6M and V12M, respectively, but also without statistically significant differences with respect to VB. Out of 11/23(47.8%) and 9/21(42.9%) moved from SLEDAI 0 to SLEDAI >0 in V6M and V12M, respectively. In terms of disease activity, no significant differences were found pre- and post-optimization in any of the measures, except for hypocomplementemia (p = 0.0276) (Table 1). Changes in activity did not lead to relevant changes in treatment, thus only one patient returned to the baseline dose of BLM and only one patient started DMARD. Significantly fewer patients received GC in V12M, even though the median dose of GC was higher in V12M (5 (0.62-8.75) vs. 2.5 (0-5) in VB) (Table 1).
Conclusion: It is possible to optimize doses of BLM without relevant changes in disease activity, at least in the short term, in a significant percentage of patients, and the most of them maintain the optimized dose. However, the increased clinical or serologic activity is possible in some patients. This makes a tighter post-optimization follow-up advisable.
More studies, with a larger number of patients and longer follow-up time, will be required to confirm these results and to analyse more robust outcomes.
To cite this abstract in AMA style:
Altabas Gonzalez I, Pego-Reigosa J, Mourino Rodriguez C, Jiménez N, Hernández-Martín A, Font J, Casafont-Sole I, Roman Ivorra J, De la Rubia Navarro M, Galindo M, SALMAN MONTE T, Narvaez J, Vidal-Montal P, García-Villanueva M, Garrote Corral S, Blázquez Cañamero M, Marras Fernández-Cid c, Piqueras García M, MARTINEZ BARRIO J, Sánchez-Lucas M, Cortés Hernández J, Penzo E, Calvo Alén J, de Dios Jiménez de Aberásturi J, Álvarez Rodríguez B, Rocha M, Tomero Muriel E, Menor-Almagro R, Gandía Martínez M, Gomez-Puerta J, Frade B, Ramos-Giráldez C, Trapero Pérez C, DIEZ ALVAREZ M, Moriano Morales C, Muñoz A, Rúa-Figueroa I. Is Belimumab Dose Optimization Possible in Patients with Systemic Lupus Erythematosus?: Analysis of This Therapeutic Strategy in a Large Multicenter Cohort of Patients from Spanish Rheumatology Departments [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/is-belimumab-dose-optimization-possible-in-patients-with-systemic-lupus-erythematosus-analysis-of-this-therapeutic-strategy-in-a-large-multicenter-cohort-of-patients-from-spanish-rheumatology-depart/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/is-belimumab-dose-optimization-possible-in-patients-with-systemic-lupus-erythematosus-analysis-of-this-therapeutic-strategy-in-a-large-multicenter-cohort-of-patients-from-spanish-rheumatology-depart/