Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: A psoriatic arthritis (PsA) core domain set to be measured in randomized controlled trials (RCT) was developed by Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and endorsed by Outcome Measures in Rheumatology (OMERACT) in 2006. Over the past 2 years, the GRAPPA-OMERACT PsA working group conducted research projects with the objective to update the PsA core domains set to reflect patients’ and physicians’ priorities and evolving PsA research.
Methods: We conducted: 1) a systematic literature review (SLR) of domains assessed in PsA RCTs and longitudinal observational studies (LOS); 2) international qualitative focus groups on five continents to identify domains important to patients with PsA; 3) international surveys with patients with PsA and physicians to prioritize domains; and 4) an international face-to-face consensus meeting among patient research partners (PRPs) and physicians using the nominal group technique (NGT) method to draft a PsA core domain set. Patient research partners (PRPs) were involved in each phase and one co-chaired the working group.
Results: Thirty-nine unique PsA domains were identified through the SLR (24 domains) and focus groups (34 domains). Patients (n=50) and physicians (n=75) completed electronic surveys rating the importance of the 39 domains for inclusion in the core set (Figure 1). At the consensus meeting, 12 patients and 12 physicians used these data to agree upon 10 domains for inclusion in PsA clinical trials, one strongly recommended to be measured at least once during the development of a new medication (middle circle) and four domains for the research agenda. These domains were rated in a second international survey with patients (n=49) and physicians (n=71) (Figure 1). The results were presented at the OMERACT conference in May 2016 and voted upon for endorsement. The updated PsA Core Domain set endorsed at OMERACT 2016 with 90% vote includes: musculoskeletal disease activity (peripheral arthritis, enthesitis, dactylitis, and spine symptoms), skin disease activity (skin and nail disease), pain, patient global, physical function, health-related quality of life, fatigue, and systemic inflammation (Figure 2).
Conclusion: The updated PsA Core Domain Set incorporates patients’ and physicians’ priorities and evolving PsA research. Next steps include identifying outcome measurement instruments that adequately assess these domains. Figure 1: Survey results from patients and physicians. 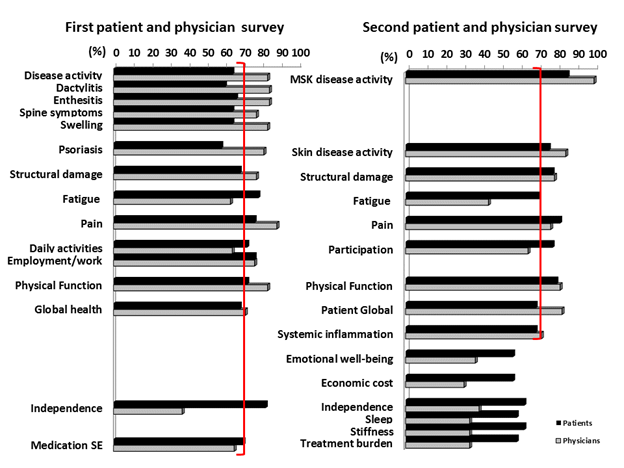 Figure 2: 2016 Psoriatic Arthritis Core Domain Set.
Figure 2: 2016 Psoriatic Arthritis Core Domain Set.
To cite this abstract in AMA style:
Orbai AM, de Wit M, Mease PJ, Shea JA, Gossec L, Leung YY, Tillett W, Elmamoun M, Callis Duffin K, Campbell W, Christensen R, Coates LC, Dures E, Eder L, FitzGerald O, Gladman DD, Goel N, Grieb S, Hewlett S, Hoejgaard P, Kalyoncu U, Lindsay C, McHugh NJ, Shea B, Steinkoenig I, Strand V, Ogdie A. International Patient and Physician Consensus on Psoriatic Arthritis Outcomes for Clinical Trials [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/international-patient-and-physician-consensus-on-psoriatic-arthritis-outcomes-for-clinical-trials/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/international-patient-and-physician-consensus-on-psoriatic-arthritis-outcomes-for-clinical-trials/

