Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: A recent consensus project (Saketkoo et al, Thorax 2014) recommended a minimum core set of outcome measures for use in future clinical trials of CTD-ILD and IPF. The World Health Organization (WHO) introduced the International Classification of Functioning, Disability, and Health (ICF) as a scientific method of disability data collection and a universal framework of >1200 categories to describe disability associated with health conditions in terms of the bio-psycho-social model with consideration of environmental and personal factors. For accurate representation of disease, ease of healthcare provision and fair allocation of resources, it is essential that ICF Core Sets be established for rare and complex diseases.
Methods: Per updated ICF linkage rules, each instrument from the published CTD-ILD and IPF core sets were deconstructed to meaningful concepts and independently linked by 2 health professionals experienced in ICF linkage (RE, LAS). Inter-linker agreement on independent linkages was analyzed (KK). Discordant linkages were resolved between linkers. A 3rd linker (OD) arbitrated if irreconcilable linkages occurred.
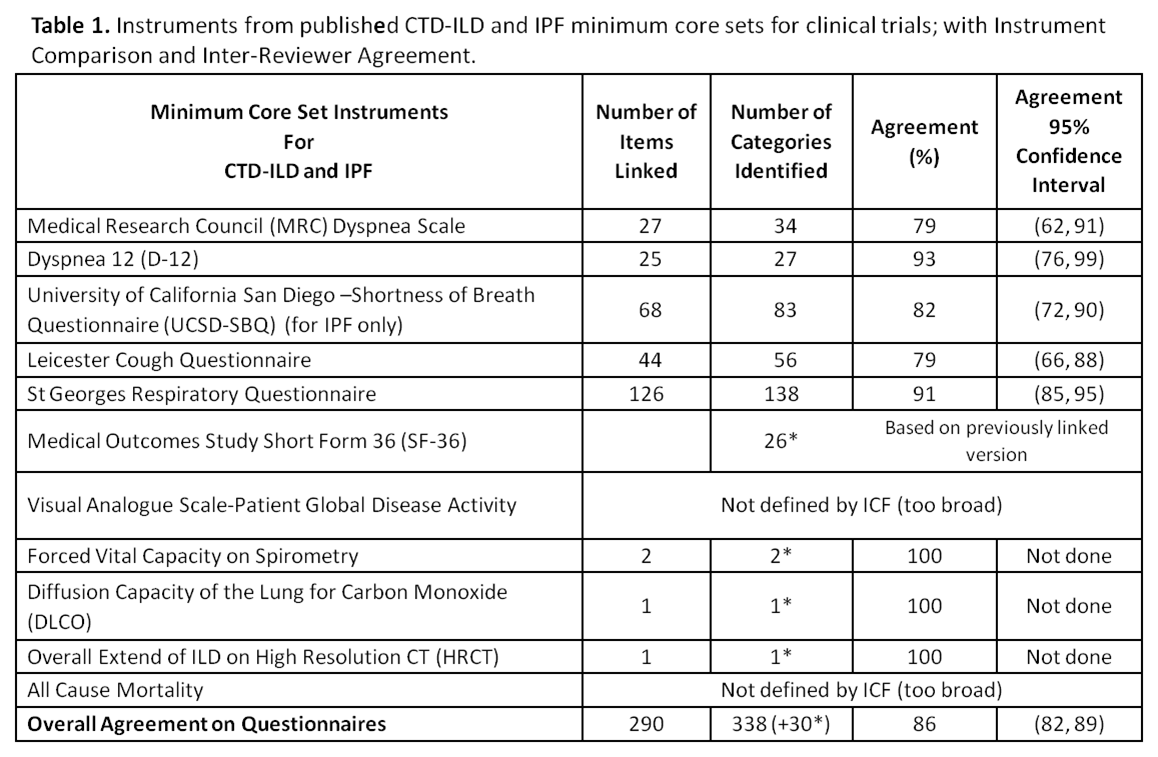
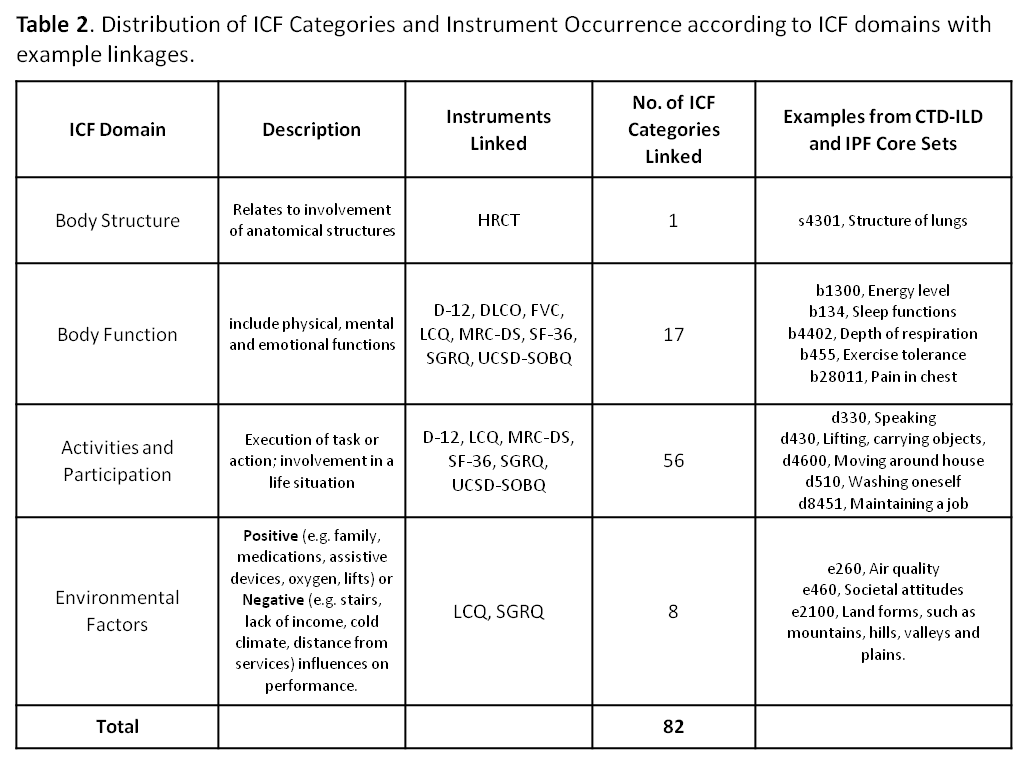
Results: Eighty-two ICF categories were identified under the 4 ICF domains for 6 patient questionnaires and 3 traditional objective measures. The proportion of agreement ranged from 0.79 (95% CI: 0.62, 0.91) to 0.93 (0.76, 0.99) (Table 1) on the 5 different survey instruments (Table 2) with the overall proportion of inter-linker agreement 0.86 (0.82, 0.89). Any discordant linkages were reconciled between the initial 2 linkers. A previously linked version of the SF-36 had already existed; All-Cause Mortality and Patient Global of Disease Activity were indefinable in ICF terms. 20 new Personal Factors were generated to capture important disease-specific qualities not elsewhere described in ICF, e.g. ‘pf_embarrassed by cough’ or ‘pf_panic/afraid when can’t get breath’.
Conclusion: This is the first effort to map CTD-ILD and IPF outcome measures to the ICF. A composite of these ICF linkages will be available to clinicians and researchers with validation studies to follow. ICF Core Sets are intended to be stream-lined disease-specific languages that support global, regional and personal health-related parity across cultures, age and socioeconomic status. ICF Core Sets enable fair assessment that may be utilized in policy making and service provision and funding. Familiarity with ICF Core Sets in CTD-ILD and IPF will enable clinicians to experience a smoother transition to ICD-11 which is under development and will meld diagnostic coding with the ICF.
Disclosure:
R. Escorpizo,
None;
K. J. Keen,
None;
K. Fligelstone,
None;
M. R. Lammi,
None;
D. LeSage,
None;
A. M. Russell,
None;
S. Birring,
None;
C. Sarver,
None;
J. Varga,
None;
O. Distler,
Actelion, Pfizer, Ergonex, BMS, Bayer, United BioSource Corporation, Roche/Genentech, medac, Biovitrium, Boehringer Ingelheim Pharma, Novartis, 4D Science, Active Biotec, Sinoxa, Sanofi-Aventis, Serodapharm, GSK, Epipharm, ,
5,
Actelion, Pfizer, Ergonex, Sanofi-Aventis,
2;
L. A. Saketkoo,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/international-classification-of-functioning-disability-and-health-icf-core-sets-for-connective-tissue-disease-interstitial-lung-disease-ctd-ild-and-idiopathic-pulmonary-fibrosis-ipf/