Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: In exploring the pathogenic mechanisms underlying neuropsychiatric lupus (NPSLE), it was discovered that cerebrospinal fluid (CSF) from lupus patients often contains neurotoxic antibodies, cytokines, and metabolites. Disruption of the blood-CSF barrier (B-CSFB), formed by choroid plexus (CP) epithelia, could explain these abnormalities. In both NPSLE patients and lupus mouse models, immune cells infiltrate the CP and can alter normal epithelial functions through extensive cytokine signaling. Interleukin-6 (IL-6) is elevated in the serum and CSF of both NPSLE patients and lupus mice. ATP-binding cassette (ABC) transporter function in the CP, including P-gp, MRP1, and BCRP, is fundamental to the clearance of neurotoxic substrates from the CSF. In this study, we assessed the effects of IL-6 on lupus mouse derived CP organoids to investigate the integrity of the B-CSFB in NPSLE.
Methods: CP tissue from 2-5 female MRL/lpr (WT) or IL-6 knockout (KO) mice of at least 16 weeks of age were pooled and cultured for two weeks to generate CP organoids for each experiment, as described (Petersen et al, 2020). Organoid morphology was confirmed using whole-mount immunostaining and electron microscopy. Gene expression was measured by real-time qPCR. A standard time-lapse permeability assay which quantified tracer fluorescence within each organoid’s central vacuole was performed under various conditions. We assessed organoid permeability to tracer in the presence of supplemental IL-6 (10 ng/mL) or anti-IL-6 receptor antibodies (1 ug/mL). We also assessed inherent tracer permeability differences between IL-6 competent WT and IL-6 KO MRL/lpr-derived organoids. The fluorescent tracers used included two small dyes, lucifer yellow (LY) and sodium fluorescein (SF), and a larger 10 kDa dextran molecule (Dex). Importantly, LY but not SF is transported by ABC transporters. Two-tail Mann-Whitney u tests compared differences between groups.
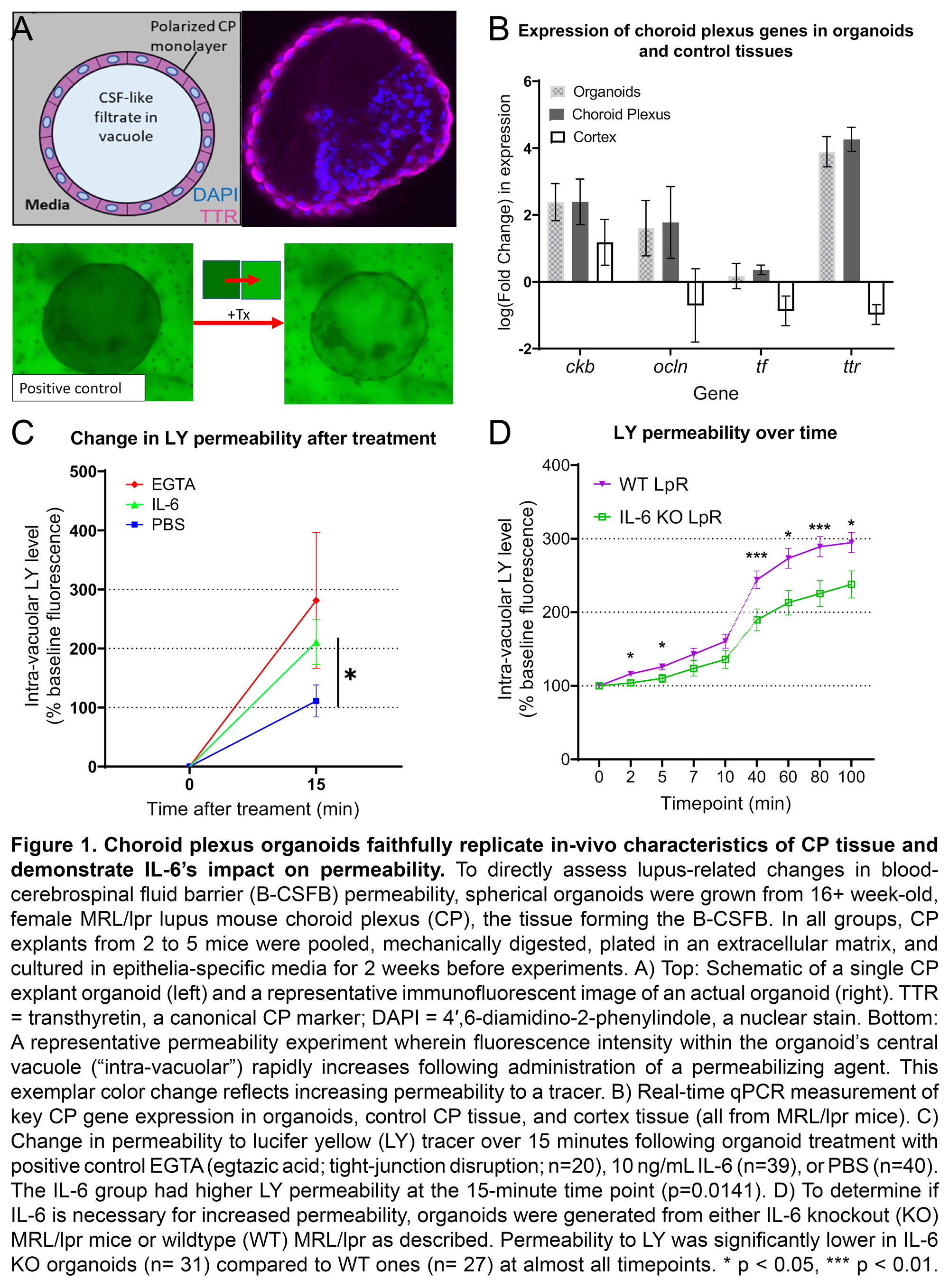
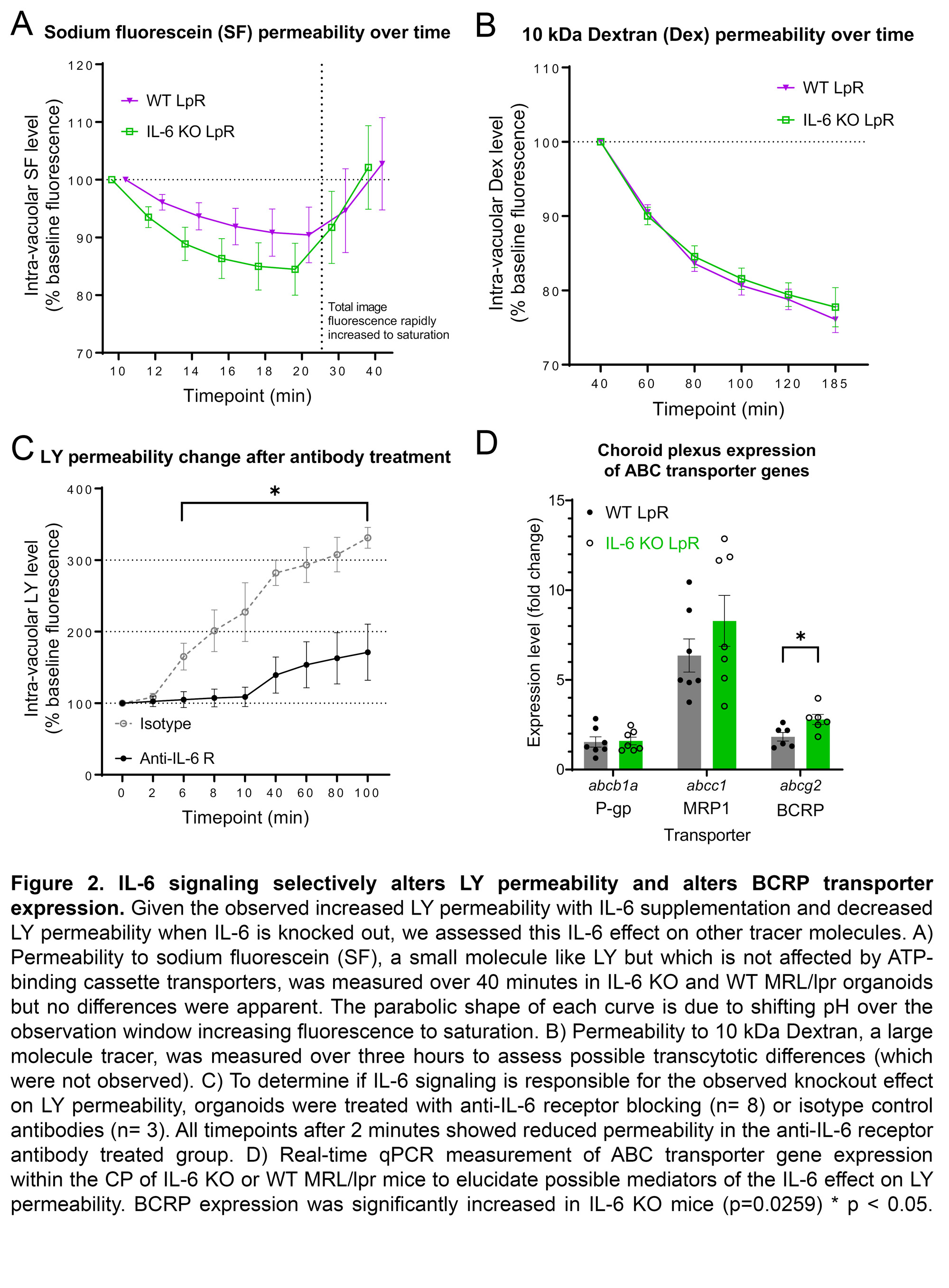
Results: MRL/lpr-derived organoids replicated the in-vivo morphology and gene expression of the CP (Fig 1A-B). IL-6 rapidly increased MRL/lpr organoid permeability to LY (IL-6: n=39, 174.4+/- 37.91; PBS: n=40, 76.1+/-27.1; p=0.014; Fig 1C). IL-6 KO organoids demonstrated consistently reduced permeability to LY compared to WT (KO: n=31; WT: n=27; p< < 0.05; Fig 1D). No permeability differences between IL-6 KO (n=19) and WT (n=19) organoids were seen for SF (Fig 2A) or Dex (Fig 2B). Incubation with an anti-IL-6 receptor antibody (n=8) significantly decreased LY permeability relative to an isotype control (n=3; Fig 2C). In-vivo expression of abcg2, a constituent of BCRP, was increased in IL-6 KO MRL/lpr mice (KO: n=6, 8.83+/-0.84; WT: n=4.17+/-0.61; p=0.026; Fig 2D).
Conclusion: In the first application of a novel CP organoid model to study lupus, we found that IL-6 signaling appears sufficient and necessary to increase permeability to LY, but not other tracers studied. Changes in BCRP, which effluxes only LY, could explain this accumulation of LY within organoids. Therefore, the high IL-6 environment of SLE could interfere with BCRP function and hinder the CP’s ability to clear potentially neurotoxic metabolites, and thus contribute to the pathogenesis of NPSLE.
To cite this abstract in AMA style:
Reynolds J, Torz L, Petersen N, Ben-Zvi A, Putterman C. Interleukin-6 Disrupts Blood-cerebrospinal Fluid Barrier Permeability in Murine Neuropsychiatric Lupus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/interleukin-6-disrupts-blood-cerebrospinal-fluid-barrier-permeability-in-murine-neuropsychiatric-lupus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interleukin-6-disrupts-blood-cerebrospinal-fluid-barrier-permeability-in-murine-neuropsychiatric-lupus/