Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Methotrexate (MTX) is now an anchor drug in the treatment strategy of rheumatoid arthritis (RA) and its effectiveness has been established. However it is still unclear how MTX should work in the pathologically complicated cytokine network in RA including tumor necrosis factor (TNF)-a and interleukin (IL)-6. The aims of this study were to reveal the kinetics of TNF-a and IL-6 during the treatment of MTX and to assess the relationship between these cytokines and clinical and radiological states.
Methods: Sixty two RA patients were enrolled in this study whose estimated disease duration before the diagnosis of RA was less than 36 months and who received MTX treatment without biologic agents for at least 3 months. Plasma IL-6 and TNF-a level were measured by CLEIA before the start of the treatment and several months after, and the clinical characteristics were gathered at the same time. Radiography assessment was performed by means of van der Heijde modified Sharp Score (mTSS). The comparison of two continuous variables was performed by Wilcoxon signed-ranks test and the correlations with two variables were analyzed by logistic and multiple regression model.
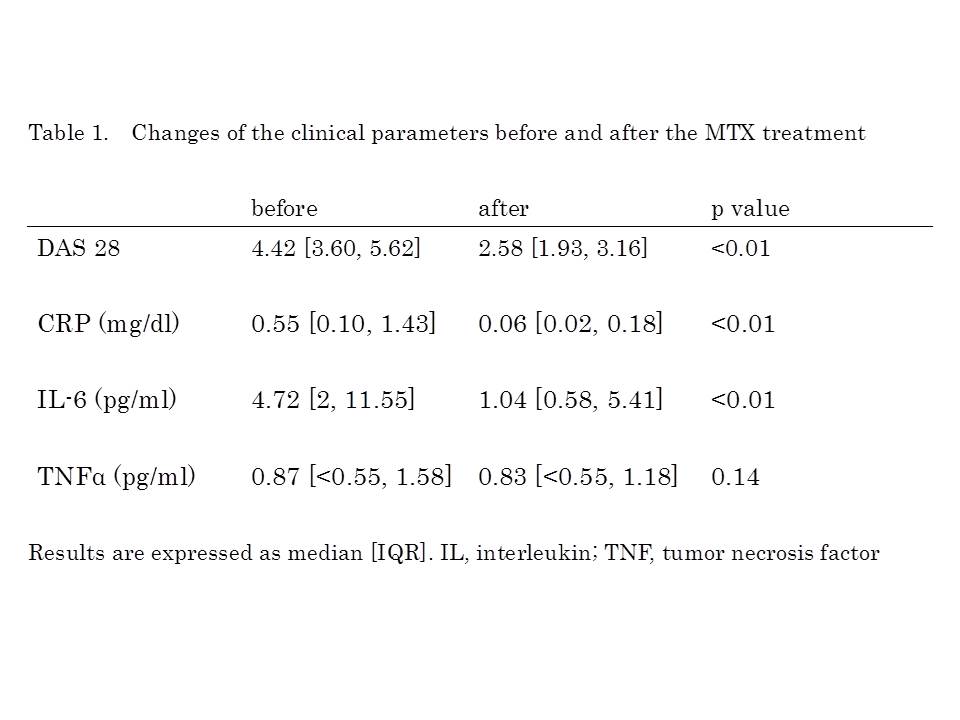
Results: Of 62 patients, 49 (79%) were female. Mean age was 57 ± 15 years and median estimated disease duration was 3 months. The time when the cytokines were measured after the start of the treatment was at a median of 11 months (5-18). Forty six patients (74%) were positive for RF and 47 patients (76%) for anti-CCP antibody. Mean MTX dosage was 8.7 ± 2.3 mg/week. With concomitant use of MTX, steroid and synthetic DMARDs were prescribed in 20 patients (prednisolone in 6 patients, bucillamine 6, salazosulfapyridine 6, bucillamine and salazosulfapyridine 1, tacrolimus 1). Compared with the values of median DAS 28, serum CRP and plasma IL-6 before the start of the treatment, those after the use of MTX were decreased significantly from 4.42 to 2.58, 0.55 to 0.06 mg/dl and 4.72 to 1.04 pg/ml, respectively (p<0.01 for all), while plasma TNF-a level didn’t change from 0.87 to 0.83 pg/ml (Table 1). Of those parameters, the estimated yearly progression of mTSS (DmTSS) was not correlated with CRP but significantly correlated with plasma IL-6 level after the use of MTX (Figure 1, R2=0.05, 0.45, respectively). In multivariate analysis, rapid radiographic progression (DmTSS≥5) was associated only with high plasma IL-6 level after the treatment [OR 1.15, 95%CI 1.02-1.32].
Conclusion: In the treatment of RA, MTX had a great effect on decreasing plasma IL-6 and the level of IL-6 after the use of MTX was the greatest impact on the radiological progression.
Disclosure:
N. Nishina,
None;
H. Kameda,
None;
Y. Kaneko,
None;
M. Kuwana,
None;
T. Takeuchi,
Abbott Japan Co., Ltd.,
2,
Astellas Pharma,
2,
Bristol-Myers K.K.,
2,
Chugai Pharmaceutical Co., Ltd.,
2,
Daiichi Sankyo Co., Ltd.,
2,
Eisai Co., Ltd.,
2,
Janssen Pharmaceutical K.K.,
2,
Mitsubishi Tanabe Pharma Co.,
2,
Nippon Shinyaku Co., Ltd.,
2,
Otsuka Pharmaceutical,
2,
Pfizer Japan Inc.,
2,
Sanofi-Aventis K.K.,
2,
Santen Pharmaceutical Co., Ltd.,
2,
Takeda Pharmaceutical Co., Ltd.,
2,
Teijin Pharma Ltd.,
2,
Astra Zeneca, K.K.,
5,
Eli Lilly Japan K.K.,
5,
Novartis Pharma K.K.,
5,
Mitsubishi Tanabe Pharma Co.,
5,
Asashi Kasai Medical K.K.,
5,
Abbot Japan Co., Ltd.,
5,
Bristol-Myers Squibb K.K.,
5,
Chugai Pharmaceutical Co., Ltd.,
5,
Eisai Co., Ltd.,
5,
Janssen Pharmaceutical K.K.,
5,
Pfizer Japan Inc.,
5,
Takeda Pharmaceutical Co., Ltd.,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interleukin-6-as-a-biomarker-for-the-clinical-and-radiological-effectiveness-of-methotrexate-in-rheumatoid-arthritis/