Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
In osteoarthritis (OA), there are no therapeutics to prevent disease progression. Canakinumab, a monoclonal antibody targeting interleukin-1β, reduced inflammation and cardiovascular events in the CANTOS trial (Ridker et al. 2017). CANTOS included 10,061 men and women with a history of myocardial infarction and high-sensitivity C-reactive protein (hsCRP) ≥2 mg/L who were randomized to placebo or one of three doses of canakinumab (50 mg, 150 mg, or 300 mg) given sc once every 3 months. Median follow-up was 3.7 years.
Methods:
We conducted a post-hoc exploratory analysis of CANTOS data to evaluate potential effects of canakinumab on rates of OA-related adverse events (AE) and incident TKR/THR (ARGUS) in the full CANTOS cohort and in a subgroup with a medical history of OA. The high-level term Osteoarthropathy (OAP) was used to search the AE database, (no adjudication of OA was pursued). A time to event analysis was done for first occurrence of TKR/THR as well as for OAP related AEs. The drug treated groups were compared to placebo by two-sided log-rank test. Second, the analyses were repeated with pooled drug treated groups and hazard ratios for pooled drug vs placebo computed by Cox proportional hazards regression (CPH).
Results:
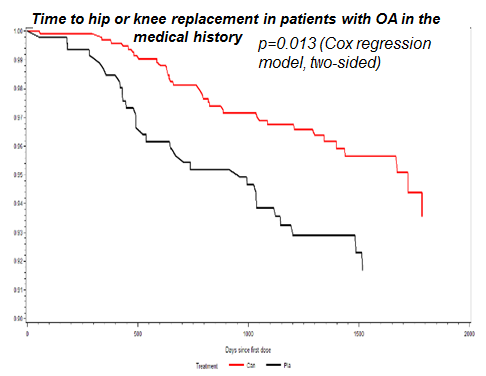
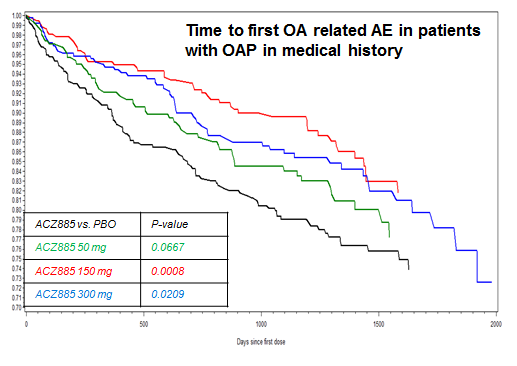
In all patients, incidence rates of TKR/THR were 1.41%, 0.88%, 0.66%, and 0.80% in the placebo and canakinumab 50 mg, 150 mg, and 300 mg groups, respectively. The relative risk reduction (RRR) for pooled treatment vs placebo was 45% computed by CPH, p<0.001. For OA related adverse events, comparable incidence rates were 7.0%, 5.2%, 5.1%, 6.0%, respectively. The RRR for pooled treatment vs. placebo was 23% computed by CPH, p=0.002. In the subgroup of 1569 CANTOS participants with OAP at baseline, incidence rates of TKR/THR were 6.25% and 3.36% in the placebo and combined canakinumab groups respectively (RRR vs placebo 45%, p<0.013, Figure 1). Incidence rates for OA related adverse events were 20.7%, 16.6%, 12.4%, 15.0%, respectively (RRR vs. placebo across groups 31%, p=0.003, Figure 2).
Conclusion:
In an exploratory analysis of the CANTOS trial, canakinumab treatment was associated with reduced rates of THR/TKR and OA symptoms. Effects were most apparent among those with a prior history of OA. An additional responder analysis based on hsCRP lowering at 3 months is currently ongoing.
References:
Ridker PM, Everett BM, McFadyen JG, et al., Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119-31
Figure 1
Figure 2
To cite this abstract in AMA style:
Schieker M, Mindeholm L, Praestgaard J, Scotti C, Solomon D, Thuren T, Dreyer K, Roubenoff R, Ridker PM. Interleukin-1β Inhibition with Canakinumab Associates with Reduced Rates of Total Hip and Knee Replacement (THR/TKR) and Osteoarthritis (OA) Symptoms: Exploratory Results from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/interleukin-1%ce%b2-inhibition-with-canakinumab-associates-with-reduced-rates-of-total-hip-and-knee-replacement-thr-tkr-and-osteoarthritis-oa-symptoms-exploratory-results-from-the-canakinumab-ant/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interleukin-1%ce%b2-inhibition-with-canakinumab-associates-with-reduced-rates-of-total-hip-and-knee-replacement-thr-tkr-and-osteoarthritis-oa-symptoms-exploratory-results-from-the-canakinumab-ant/