Session Information
Session Type: Abstract Submissions (ACR)
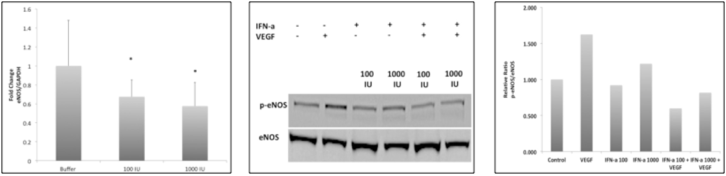
Background/Purpose: Premenopausal SLE patients have a devastating increase in cardiovascular disease (CVD) and major associated cardiovascular events (MACE) that are not fully explained by Framingham risk factors. Recent in vitro studies suggest that Type I interferons promote endothelial progenitor cell dysfunction and apoptosis while others have shown that Type I interferon gene signature correlates with increased endothelial dysfunction (ED) in SLE. Although causes of ED are likely multifactorial, all pathways converge on the diminished activity of endothelial nitric oxide synthase (eNOS). The low levels of nitric oxide (NO) produced by eNOS has anti-inflammatory, anti-thrombotic, and anti-vasoconstrictive properties all important in preventing atherosclerosis. We examined the effects of IFN-alpha on eNOS gene expression and phosphorylation. Methods: Human umbilical vein endothelial cells (HUVECs) were cultured and treated as described below. Changes in eNOS expression in response to IFN-α (100, 1000 IU) were quantified by real-time PCR. Functional changes in response to vascular endothelial growth factor (VEGF 50ng/ml, 30 minutes) were assessed by immunoblot. We also evaluated the effect of serum from patients that induce a type I IFN signature (induction of two inducible genes (MX1 and IFIT1); IFIGs) from 5 patients and 2 controls on eNOS expression and VEGF mediated function. Gene expression was assessed using RT2PCR and phosphorylated serine 1177 eNOS expression using immunoblot. Type I IFN neutralization studies (IFN-α antibody(Ab), IFNα receptor (IFNAR) Ab) were also performed and eNOS expression was assessed. Results: HUVECs treated with IFN-α at 100IU and 1000IU exhibited a significant reduction in eNOS gene expression at 24 hours (33% and 42%, respectively) and a reduction in VEGF-induced phosphorylation at the serine 1177 site (64% and 50%, respectively) after 24 hours. SLE serum also caused a 40% reduction in eNOS gene expression in human aortic endothelial cells (HAECs) compared to normal serum. However, addition of IFN neutralizing antibodies to serum did not reverse observed effects on eNOS gene changes. Conclusion: SLE patients are at an increased risk for vascular disease, and 40% of patients present with IFN signature gene expression. Recent studies suggest that Type I IFNs are important for prediction of vascular endothelial function in SLE patients. Endothelial nitric oxide synthase (eNOS) protects the endothelium from damage and our preliminary data suggest that IFNα may have detrimental effects on eNOS expression and function. Thus, a rational target for the effect of IFNα on endothelial function may be the downstream effects of IFNα on eNOS gene expression and protein activation.
Disclosure:
J. Buie,
None;
J. Oates,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interferon-alpha-decreases-endothelial-nitric-oxide-synthase-function-and-expression-in-human-umbilical-vein-endothelial-cells/