Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
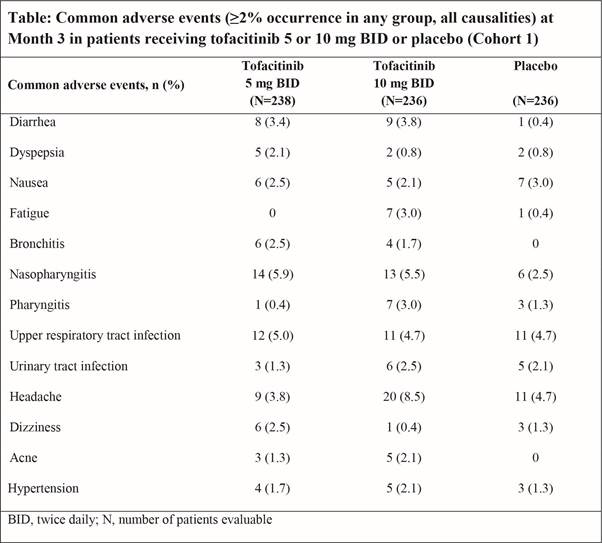
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor under investigation for psoriatic arthritis (PsA). We describe the safety profile of tofacitinib from integrated Phase (P)3 and long-term extension (LTE) studies.
Methods: Data were analyzed for patients (pts) who received ≥1 dose of tofacitinib 5 or 10 mg twice daily (BID) or placebo (PBO), integrated across 2 P3 studies (OPAL Broaden [12 months; NCT01877668]; OPAL Beyond [6 months; NCT01882439]) and 1 LTE study (OPAL Balance [ongoing, database not locked; NCT01976364]). Common adverse events (AEs; occurring in ≥2% of tofacitinib pts in any group) were analyzed in the PBO-controlled portion (Months 0–3) of the P3 studies (Cohort 1 [C1]). Serious AEs (SAEs) and discontinuations due to AEs were analyzed over 12 months in pts randomized to tofacitinib 5 or 10 mg BID in P3 studies (Cohort 2a [C2a]); pts randomized to PBO were excluded from this analysis. Deaths and AEs of special interest (serious infections [SI], herpes zoster [HZ], opportunistic infections [OI] including HZ, major adverse cardiovascular events [MACE], malignancies, non-melanoma skin cancer [NMSC]) were evaluated in all tofacitinib-treated pts in the P3 and LTE studies (Cohort 3 [C3]). Incidence rates (IR; pts with events/100 pt‑years [PY] and 95% confidence intervals) are reported. Laboratory results will be reported in future publications.
Results: C1 included 474 tofacitinib- and 236 PBO-treated pts; C2a included 474 tofacitinib-treated pts; and C3 included 783 tofacitinib-treated pts (exposure: 776 PY). Nasopharyngitis (5.9%) and headache (8.5%) were the most commonly reported AEs at Month 3 in pts receiving tofacitinib 5 and 10 mg BID, respectively (Table). In pts randomized to tofacitinib 5 or 10 mg BID, over 12 months (C2a), the IRs for SAEs were 7.92 (4.09, 13.84) and 8.11 (4.19, 14.17), respectively. Discontinuation due to AEs occurred in 11 (4.6%) and 11 (4.7%) pts randomized to tofacitinib 5 and 10 mg BID, respectively, with IRs of 7.16 (3.58, 12.82) and 7.31 (3.65, 13.08), respectively, over 12 months (C2a). Across all tofacitinib-treated pts in the P3 and LTE studies (C3), SIs occurred in 11 pts (1.4%; IR 1.40 [0.70, 2.50]). HZ was reported in 16 pts (2.0%; IR 2.05 [1.17, 3.33]) receiving tofacitinib. All 3 cases of multidermatomal HZ were adjudicated as OIs; these were the only OIs (0.4%; IR 0.38 [0.08, 1.11]). In C3, 2 deaths occurred (0.3%; IR 0.25 [0.03, 0.91]); all were considered unrelated to the study drug. MACE were reported in 3 pts (0.4%; IR 0.38 [0.08, 1.11]), malignancies (excluding NMSC) in 5 pts (0.6%; IR 0.63 [0.21, 1.48]), and NMSC in 4 pts (0.5%; IR 0.51 [0.14, 1.30]) in C3.
Conclusion: Tofacitinib in active PsA demonstrated a safety profile consistent to that seen with tofacitinib in RA; no new risks were identified. Longer-term follow-up and larger pt populations will provide further information on the safety profile of tofacitinib in pts with PsA.
To cite this abstract in AMA style:
Burmester GR, FitzGerald O, Winthrop K, Azevedo VF, Rigby WFC, Kanik KS, Wang C, Biswas P, Jones T, Menon S, Palmetto N, Rojo R. Integrated Safety Summary of Tofacitinib in Psoriatic Arthritis Clinical Studies [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/integrated-safety-summary-of-tofacitinib-in-psoriatic-arthritis-clinical-studies/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrated-safety-summary-of-tofacitinib-in-psoriatic-arthritis-clinical-studies/