Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: PsA
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: JAK-inhibitors (JAKi) are increasingly being prescribed to treat various inflammatory conditions, including psoriatic arthritis (PsA). While the understanding of JAKi efficacy and safety in rheumatoid arthritis is progressing, it remains less developed for PsA. The purpose of this study was to evaluate the profile of PsA patients to whom JAKi are prescribed, in a large multi-country real-world collaboration (JAK-pot).
Methods: Patients with a diagnosis of PsA, treated with either JAKi, TNF-inhibitors (TNFi) or bDMARDs with other modes of action (OMA) were included from 10 registers in which JAKi were prescribed for PsA. Treatment-courses were included only since JAKi became available in each country. We used standard descriptive statistics to evaluate patient-, disease-, and treatment characteristics across treatment groups, and across registers for JAKi only. We plotted crude retention rates for each treatment group.
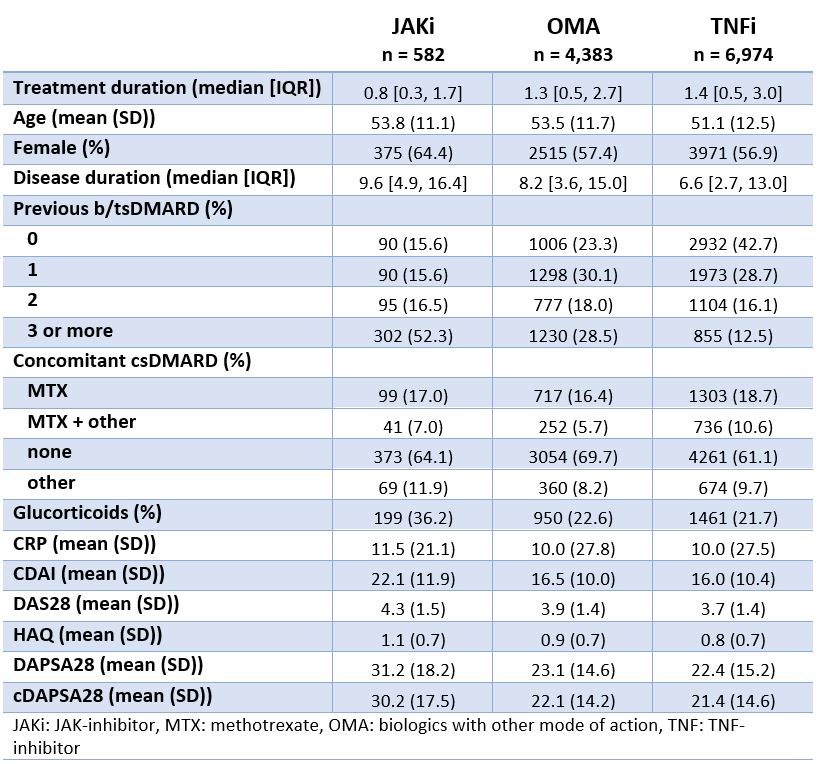
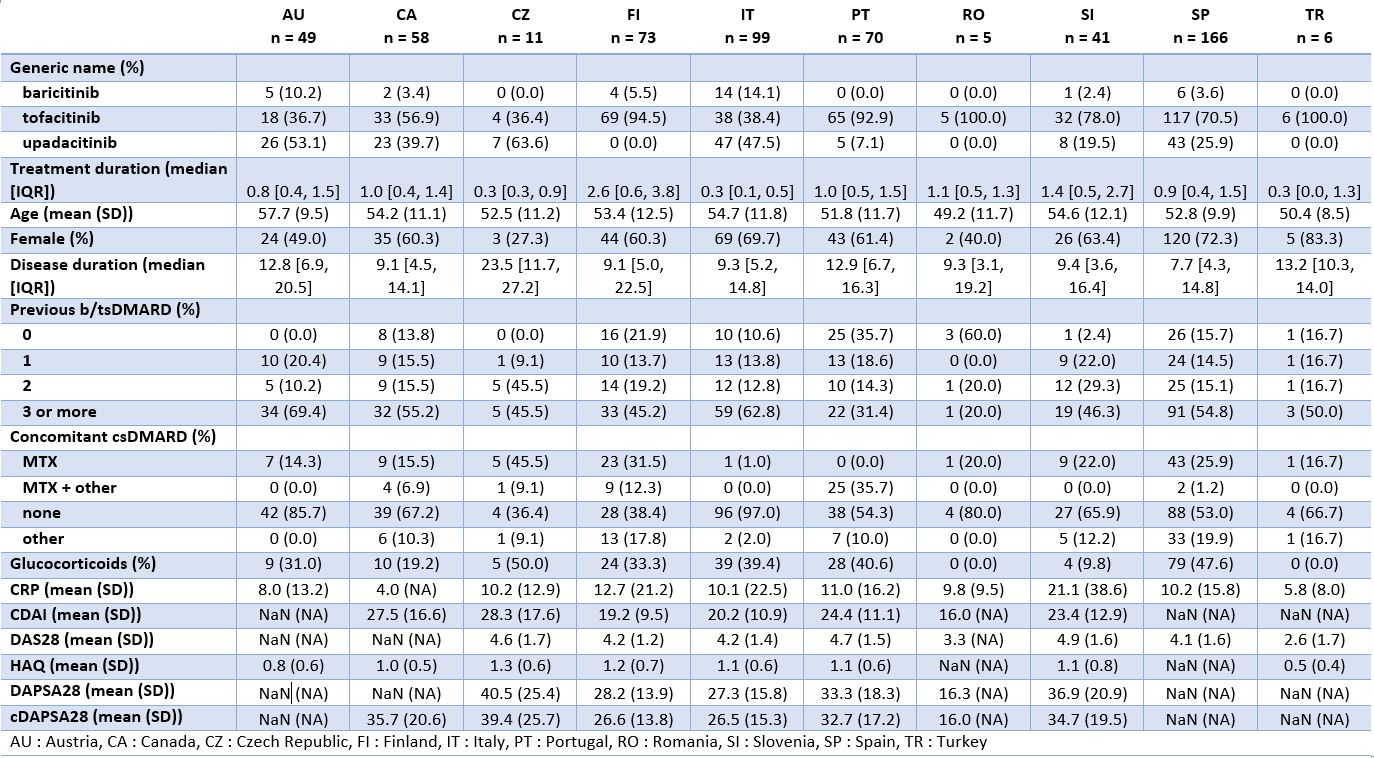
Results: Among the 11,939 treatment courses considered (Table 1), 582 were JAKi, mainly tofacitinib (67%) and upadacitinib (27%), and to a lesser extent baricitinib (6%). Patients initiating JAKi tended to have more difficult to treat disease, defined as longer disease duration ( > 9 years), older age, more prior bDMARD experience (52% with 3 or more previous bDMARDs), and more concomitant glucocorticoids. JAKi patient characteristics were consistent across countries (Table 2), despite varying use of specific JAKi agents, tofacitinib use ranging from 36 to 100% and upadacitinib from 0 to 64%. Crude drug retention at 1 year was 65% for JAKi was (Figure 1), which was significantly lower than for OMA (74%) and TNFi (77%).
Conclusion: Unadjusted drug retention rates for second line therapies in PsA patients suggest lower drug maintenance of JAKi compared to OMA and TNFi. However, it is likely that these results are largely driven by the severity of the disease of patients on JAKi compared to patients on other treatments. Adjusted analyses are needed when evaluating the real-life effectiveness and safety of JAKi for PsA patients.
To cite this abstract in AMA style:
Aymon R, Mongin D, Leeb B, Mustak-Blagusz M, Závada J, Pavelka K, Nordstrom D, Trokovic N, Iannone F, Codreanu C, Rotar Z, Kvien T, Provan S, Pombo Suarez M, Alonso F, Onen F, Inanc N, Coupal L, Choquette D, Lukina G, Elkayam O, Furer V, Rodrigues A, COURVOISIER D, Finckh A, Nissen M, Lauper K. Insights on the Use of JAK-inhibitors in Patients with Psoriatic Arthritis in an International Collaboration of Registers (the “JAK-pot” Study) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/insights-on-the-use-of-jak-inhibitors-in-patients-with-psoriatic-arthritis-in-an-international-collaboration-of-registers-the-jak-pot-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/insights-on-the-use-of-jak-inhibitors-in-patients-with-psoriatic-arthritis-in-an-international-collaboration-of-registers-the-jak-pot-study/