Session Information
Date: Tuesday, November 12, 2019
Title: Pediatric Rheumatology – ePoster III: Systemic JIA, Fever, & Vasculitis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The effectiveness of IL-1 and IL-6 inhibitors as first-line therapy in patients with new-onset systemic JIA has led to the concept of a “window of opportunity” in systemic JIA in which early initiation of biologic therapy improves outcomes. Despite this, provider surveys indicate there is significant variation in treatment with biologic therapy at diagnosis. We leveraged encounter data from the Pediatric Health Information System (PHIS) to describe treatment patterns in a large inpatient multi-site cohort of new-onset systemic JIA patients.
Methods: Using the PHIS de-identified data from inpatient encounters across 45 tertiary care children’s hospitals across the United States, we identified the index admission for children age ≤ 18 years with a discharge ICD-10 code for systemic JIA (M08.2*) between October 1, 2015 and June 1, 2018. Index admission was defined as the first admission during the inclusion period with the diagnosis code of M08.2* excluding patients with a prior admission with this code or an ICD-9 code for systemic JIA (714.30) in ≥ 1 year prior. Patients were excluded if 1) there was no administration of a conventional or biologic disease-modifying drug, glucocorticoids (GCs), or Nonsteroidal Anti-inflammatory Drugs (NSAIDs), 2) biologic or GC exposure occurred on the day of admission or hospital day 2, or 3) the primary discharge diagnosis was infection. Mixed-effects logistic regression was used to determine patient and hospital level predictors of treatment with admission hospital included as a random effect to account for clustering. Variation in treatment over time was analyzed using an extension of the Wilcoxon rank-sum test for trends.
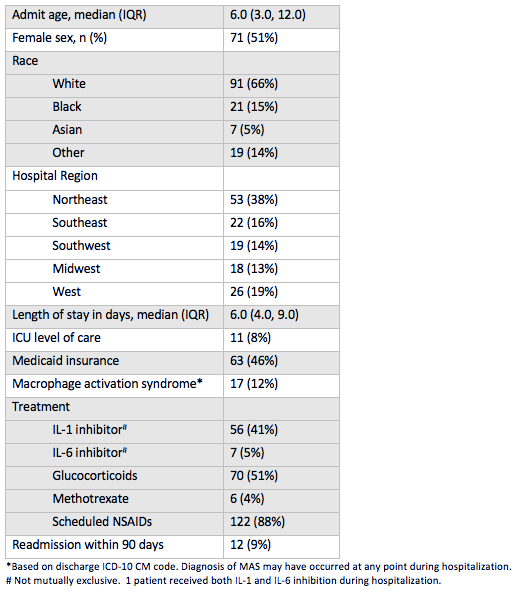
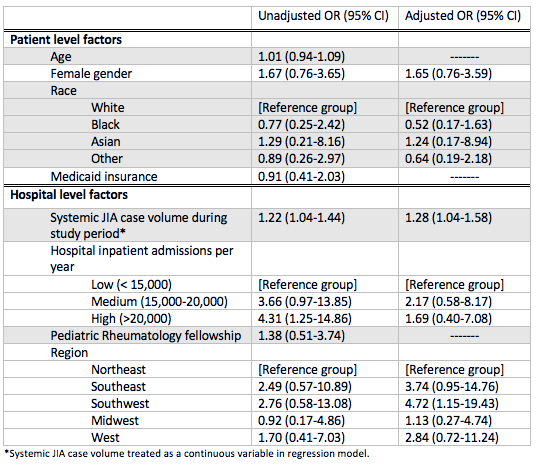
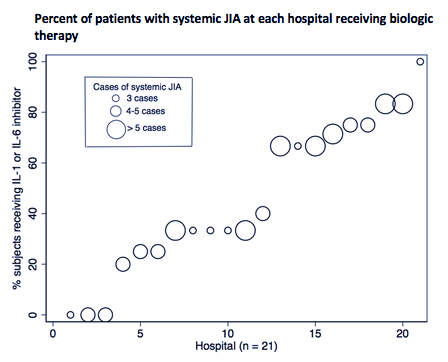
Results: A total of 138 children with new-onset systemic JIA were admitted across 45 children’s hospitals, of which 46% received biologic therapy (89% IL1i, 11% IL6i) and 51% received GCs. Demographics and clinical characteristics are presented in Table 1. The average length of stay was 6 days (IQR 4-9) and all-cause 90-day readmission rate was 9%. After adjustment for patient and hospital level factors, site systemic JIA volume was a positive predictor of biologic exposure (OR 1.28, [1.04-1.58]) (Table 2). Predictors of GC exposure included low hospital volume (OR 30.40, [5.40-171.03]), West region (OR 11.91, [1.89-74.92]), and on-site pediatric rheumatology fellowship (OR 4.45, [1.13-17.55]). Biologic exposure increased over time (p = 0.03), while GC exposure remained unchanged (p = 0.85). The median proportion of patients with GC and biologic exposure within sites was 0.67 (IQR 0.2-1) and 0.33 (IQR 0-0.67), respectively. Variability in the unadjusted use of biologics across hospitals is shown in Figure 1.
Conclusion: Treatment of children with new-onset systemic JIA varies considerably between tertiary care children’s hospitals in the United States. Patients admitted to sites with a higher number of systemic JIA cases were more likely to be exposed to biologics, whereas GC exposure was more likely at lower volume hospitals. GC use has remained unchanged over time despite increasing biologic use. Further study is needed to determine the impact of early biologic use on hospital outcomes and contributors to low biologic use and high GC use at certain sites.
To cite this abstract in AMA style:
Peterson R, Xiao R, Katcoff H, Fisher B, Weiss P. Inpatient Treatment Variation in New-Onset Systemic Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/inpatient-treatment-variation-in-new-onset-systemic-juvenile-idiopathic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inpatient-treatment-variation-in-new-onset-systemic-juvenile-idiopathic-arthritis/