Session Information
Session Type: Abstract Submissions (ACR)
Initiation of Tumor Necrosis Factor Alpha (TNFα) antagonists and risk of fractures in patients with rheumatoid arthritis (RA)
Background/Purpose: Inflammation mediates bone resorption, in part through TNFα. In experimental models TNFα antagonists reduce inflammation-mediated bone resorption; however, the effect of this class of drugs on fracture risk in patients is unknown. We tested the hypothesis that initiation of TNFα antagonists reduced the risk of fractures compared to nonbiologic comparators in patients with RA.
Methods: Using four large administrative databases we assembled a retrospective cohort of patients with RA from 1998 to 2005 enrolled in Tennessee’s Medicaid Program (TennCare), Kaiser Permanente Northern California (KPNC), Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE), and multi-State Medicaid programs (MAX) and identified patients who initiated either a TNFα antagonist (n=20,814) or a non biologic disease modifying anti-rheumatic drug (DMARD): hydroxychloroquine (HCQ), sulfazalasine (SSZ) and/or leflunomide (LEF) (n=8,964). We used baseline covariate data to calculate propensity scores (PS) to match treatment groups, and Cox regression to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs). We compared the risk of the first fracture (hip, radius/ulna, humerus, or pelvic); first hip fracture; and first clinical vertebral fracture between PS-matched cohorts of new users of TNFα antagonists and non biologic DMARDs.
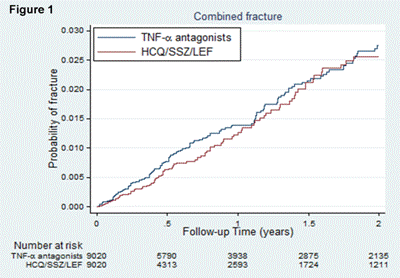
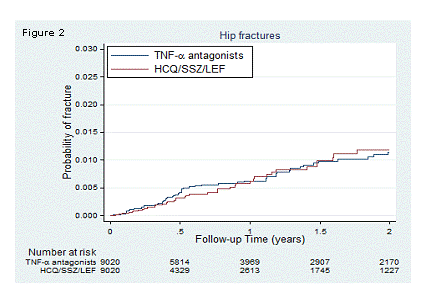
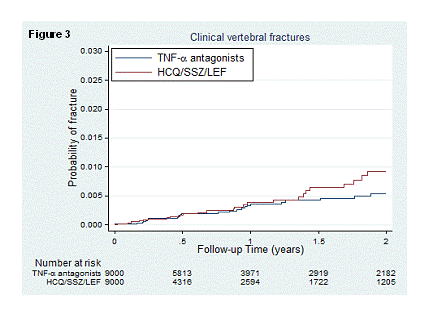
Results: We identified 9,020 new PS matched episodes of TNFα antagonist and non biologic DMARD use. The risk of fractures was similar between new users of TNFα antagonists and non biologic DMARDs: HR:1.17, 95%CI [0.91-1.51] for combined fracture outcome (Figure 1); HR:0.87, 95%CI [0.60-1.27] for hip fracture (Figure 2); and HR:0.71, 95%CI [0.43-1.19] for clinical vertebral fracture (Figure 3). The risk of the combined fracture outcome was associated with an average daily dose of prednisone equivalents >10 mg/day at baseline compared with no glucocorticoid (HR: 1.54, 95%CI [1.03, 2.30]).
Conclusion: The risk of fracture did not differ between new users of TNFα antagonists and non biologic DMARDs in patients with RA. The use of >10mg/day of prednisone equivalents at baseline increased the fracture risk.
Disclosure:
V. K. Kawai,
None;
C. Grijalva,
None;
P. Arbogast,
None;
J. R. Curtis,
Roche/Genetech, UCB, Centocor, CORRONA, Amgen Pfizer, BMS, Crescendo, Abbott,
5,
Roche/Genetech, UCB, Centocor, CORRONA, Amgen Pfizer, BMS, Crescendo, Abbott,
2;
D. H. Solomon,
Amgen & Lilly,
2,
Corrona,
5,
Pfizer Inc,
9;
E. S. Delzell,
Amgen Inc,
2;
L. Chen,
None;
L. Herrinton,
Procter and Gamble, Centocor,Genentech,
2;
L. Liu,
None;
E. F. Mitchell Jr.,
None;
C. M. Stein,
None;
M. Griffin,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/initiation-of-tumor-necrosis-factor-alpha-tnf%ce%b1-antagonists-and-risk-of-fractures-in-patients-with-selected-rheumatic-and-autoimmune-diseases/