Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Low body mass index (BMI)
predicts adverse outcomes in rheumatoid arthritis (RA), in part due to weight
loss among patients with severe disease and comorbid illness. Weight loss is a
strong predictor of adverse long-term outcomes including death. We examined how
common therapies influence short-term changes in BMI in a large database of patients
with RA.
Methods: We used 3 administrative VA database sources:
the Corporate Data Warehouse (CDW), the Decision Support System (DSS) National
Pharmacy Extract, and the Pharmacy Benefits Management (PBM) database. Among patients with at least one diagnosis code for
RA, algorithms integrated sources to define unique dispensing episodes of
methotrexate, prednisone, leflunomide, and Tumor Necrosis Factor inhibitors
(TNFi). The closest values for CRP and BMI within 30 days of the treatment
start date were used as baseline values. CRP and BMI values 90, 180, and 365
days from the treatment start date (+/- 30 days) were recorded. Important weight
loss was defined as a decrease in BMI of more than 1 kg/m2. Regression
models evaluated changes in BMI at 6-months among treatments compared to
methotrexate adjusting for potential confounders such as demographics,
seropositivity, CRP, 6-month change in CRP, disease duration, current smoking, and
comorbid conditions, including the Rheumatology Disease Comorbidity Index
(RDCI). Matched weighting on propensity was utilized adjust for confounding by
indication. Sensitivity analyses considered dual use of other RA therapies.
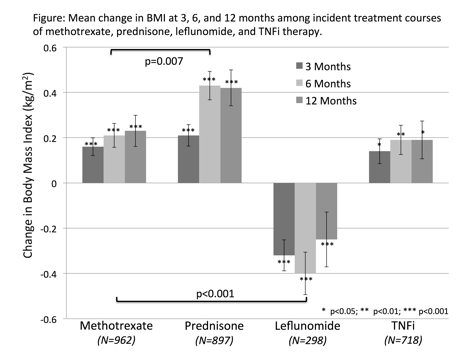
Results: There were 45,264 unique incident treatment
courses in 31,175 patients with available BMI data. There were differences in
patient characteristics between those receiving different treatments. Prednisone-treated
patients had more weight gain, while those receiving leflunomide had
substantially greater weight loss (p<0.001) (Figure). In multivariable
models there was more weight loss among leflunomide users compared to
methotrexate [β: -0.40 (-0.45, -0.35) p<0.001] and more weight
gain among prednisone users [β: 0.072 (0.042, 0.10)
p<0.001] while no differences were observed with TNFi. There was a greater risk
of weight loss for leflunomide after adjustment [OR 1.73 (1.55, 1.79)
p<0.001]. Associations between therapies and weight change were not
attenuated with propensity-adjustment or in sensitivity analyses. Predictors of
weight loss included older age, greater CRP, no improvement in CRP, greater
BMI, current smoking, disease duration, RDCI, lung disease, and malignancy.
Weight loss was associated with drug discontinuation at 6 months and death at 3
years.
Conclusion: Patients initiating leflunomide are marginally
(75%) more likely to lose a clinically significant amount of weight compared to
those who start other therapies after adjusting for confounding factors. Prednisone
use is independently associated with modest weight gain.
To cite this abstract in AMA style:
Baker J, Sauer B, Michaud K, Cannon GW, Ibrahim S, Caplan L, Davis LA, Cannella AC, Mikuls TR. Initiation of Disease Modifying Therapies and Subsequent Weight Change in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/initiation-of-disease-modifying-therapies-and-subsequent-weight-change-in-rheumatoid-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/initiation-of-disease-modifying-therapies-and-subsequent-weight-change-in-rheumatoid-arthritis/