Session Information
Date: Tuesday, November 9, 2021
Title: Abstracts: Measures & Measurement of Healthcare Quality (1893–1896)
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: Hydroxychloroquine (HCQ) is a commonly used medication for patients with lupus erythematosus, rheumatoid arthritis, and other autoimmune conditions. However, HCQ daily doses of ≥ 5 mg/kg/day can cause retinal toxicity with long-term use. We developed and piloted a medication safety dashboard to improve safe prescribing of HCQ.
Methods: The dashboard was created using Power BI (Microsoft), a data management software package available within the Veterans Health Administration (VHA) for approved users with secure access to EHR data. We extracted values for patients’ most recently prescribed HCQ dose and body weight to calculate HCQ dose in mg/kg/day. The dashboard displayed patient identifiers, HCQ dose with a flag if ≥ 5.2 mg/kg/day, and prescriber name and service. It also displayed national-, facility-, and prescriber-level performance (proportion of patients with inappropriate HCQ doses/total number of patients receiving HCQ) for benchmarking (Figure 1).
We enlisted providers from 6 VHA pilot sites to test the dashboard based on their willingness to participate and sub-optimal facility-level performance on HCQ dosing ( < 75th percentile nationally). We used Xbar-R control charts to assess the proportion of patients with inappropriate HCQ doses over 7 months. Pilot site leaders (N=7) were asked to provide qualitative feedback on the dashboard’s usability, features, and the likelihood of recommending it to others via an online survey (Survey Monkey).
Results: There were 18,635 users of HCQ nationwide across the VHA. 80% of HCQ users had a rheumatology visit within the past 5 years. 1,365 were included at the 6 pilot sites. Pilot site performance improved from 11.7% to 9.8% by May 2021. The Xbar-R control chart showed a significant improvement in the proportion of patients with inappropriate HCQ doses over this 6-month period (Figure 2). Non-participating sites’ performance was stable within the chart’s control limits from November 2020 to May 2021 (16.0-15.8%).
6 leaders from 5 pilot sites completed the user feedback survey. 5 out of 6 reported the dashboard to be easy to use and would recommend the dashboard to a trainee or colleague. Respondents reported using the dashboard for quality improvement projects and as part of local pay-for-performance programs.
Conclusion: Use of a medication safety dashboard to improve safe prescribing of HCQ resulted in significant improvement across 6 pilot sites over a 6-month period. With continued feedback from pilot sites, we plan to disseminate the dashboard to VHA clinics nationwide and evaluate performance on safe HCQ dosing over time. Rapid deployment of EHR-based dashboards can facilitate quality improvement by providing key measures and actionable reports directly to clinicians.
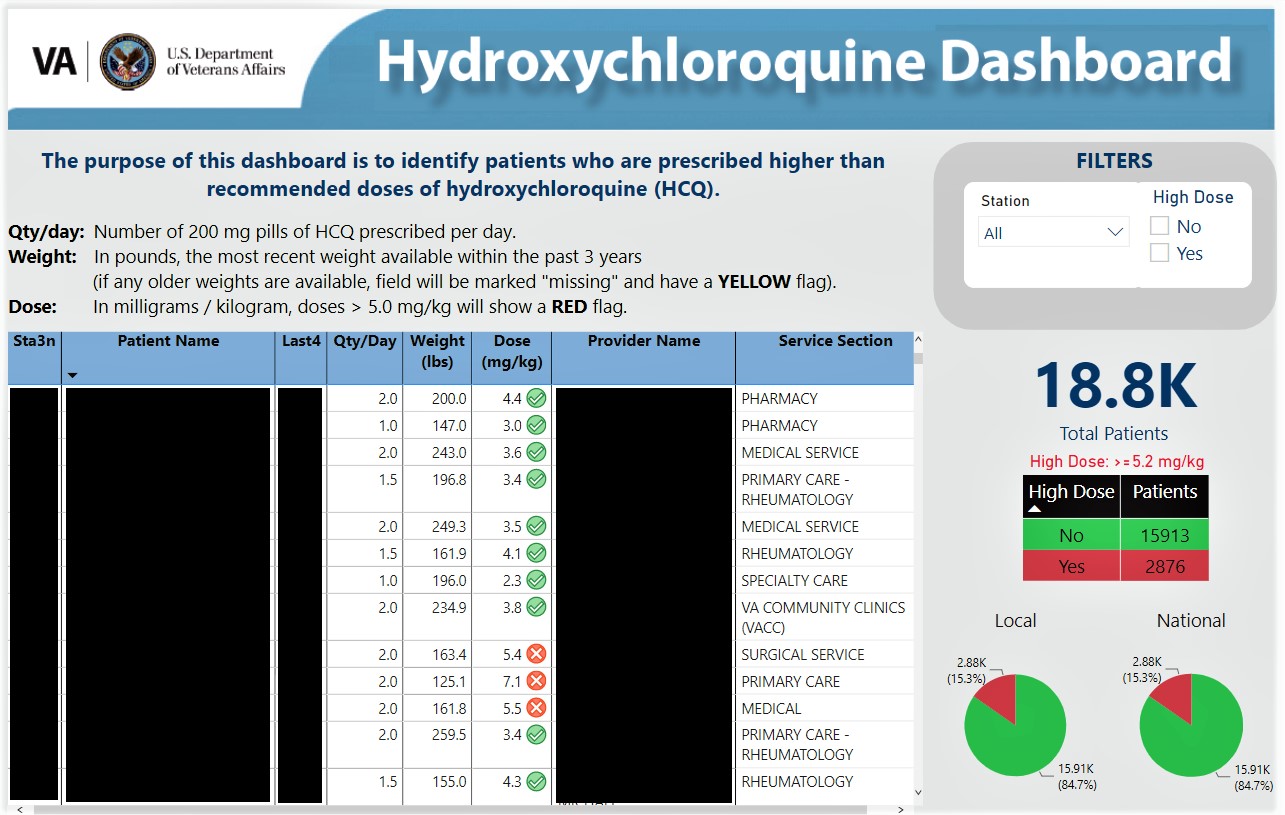 Hydroxychloroquine Dashboard (PHI removed).
Hydroxychloroquine Dashboard (PHI removed).
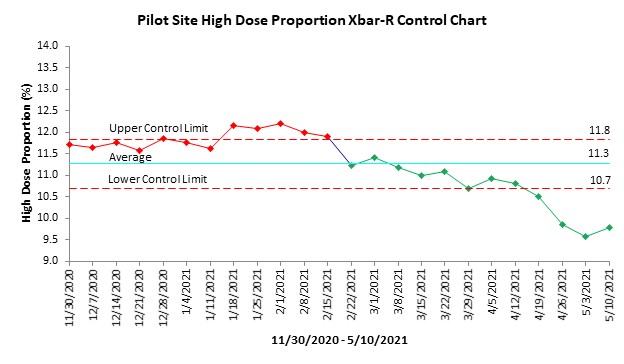 Bar-R Control Chart showing mean proportion of patients receiving inappropriate HCQ doses (≥ 5.2 mg/kg/day) across six pilot sites. X-axis shows 2-week time segments during the study period; y-axis shows proportion of patients with an active prescription for HCQ who were receiving inappropriate doses. Turquoise and red-dotted lines show the average and upper and lower control limits. Red and green dots represent performance at baseline and after a change in a stability trend, respectively.
Bar-R Control Chart showing mean proportion of patients receiving inappropriate HCQ doses (≥ 5.2 mg/kg/day) across six pilot sites. X-axis shows 2-week time segments during the study period; y-axis shows proportion of patients with an active prescription for HCQ who were receiving inappropriate doses. Turquoise and red-dotted lines show the average and upper and lower control limits. Red and green dots represent performance at baseline and after a change in a stability trend, respectively.
To cite this abstract in AMA style:
Montgomery A, Tarasovsky G, Ehiorobo I, Whooley M, Barton J, Sheth K, Reiter K, Keller M, Chung L, Bennett L, Dana J, Wahl E, Schmajuk G. Initial Results from the Implementation of a National Hydroxychloroquine Safe Prescribing Dashboard Within the Veterans Health Administration [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/initial-results-from-the-implementation-of-a-national-hydroxychloroquine-safe-prescribing-dashboard-within-the-veterans-health-administration/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/initial-results-from-the-implementation-of-a-national-hydroxychloroquine-safe-prescribing-dashboard-within-the-veterans-health-administration/
