Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with active psoriatic arthritis (PsA) are at risk of irreversible joint damage that may significantly impact quality of life. Guselkumab (GUS), a fully human monoclonal antibody that selectively targets IL-23p19 subunit and shows enhanced potency for inhibiting IL-23 signaling, has demonstrated efficacy and a favorable safety profile in patients with active PsA. The study reported findings through Week (W)24 of the ongoing phase 3b, randomized, double-blind, placebo (PBO)-controlled APEX study (NCT04882098), aimed at further evaluating GUS effects on clinical and radiographic outcomes in participants (pts) with active PsA.
Methods: APEX enrolled biologic-naïve adults with active PsA (≥3 tender and ≥3 swollen joints; C-reactive protein ≥0.3 mg/dL) and ≥2 joints with erosions on radiographs of hands and feet, despite previous non-biologic DMARDs, apremilast, or NSAIDs. Pts were randomized 5:7:7 to subcutaneous GUS 100mg Q4W; GUS 100mg at W0, W4, then Q8W; or PBO Q4W. The primary and major secondary endpoints were: 1) proportion of pts achieving ≥20% improvement in ACR response criteria (ACR20); 2) mean change from baseline (BL) in PsA-modified van der Heijde-Sharp (vdH-S) score (average of 2 readers blinded to chronological order), respectively, at W24 (both multiplicity-controlled for each GUS regimen vs PBO). Efficacy analyses include all randomized pts except those from Ukrainian sites unable to support key study operations (modified full analysis set [mFAS], Nf1020); safety analyses include all pts who received ≥1 dose of study treatment through W24 (Nf1054).
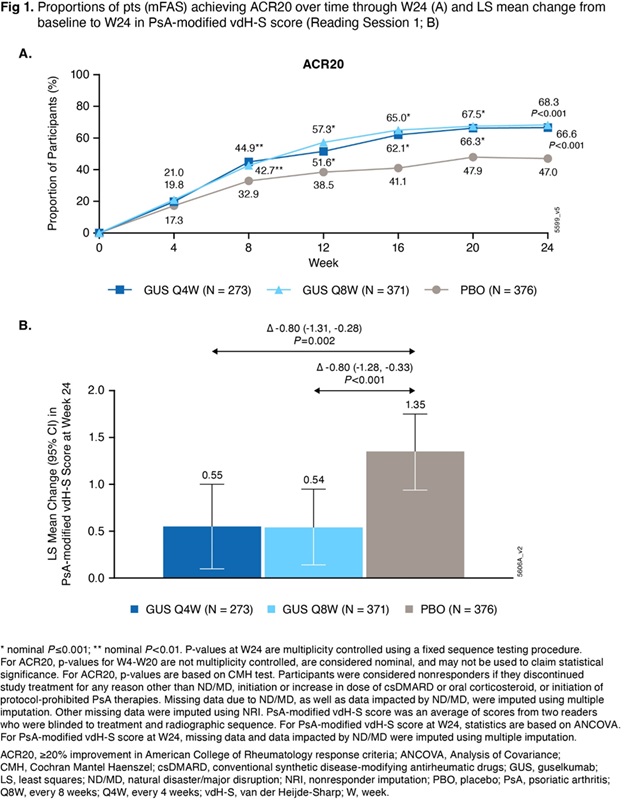
Results: In mFAS (Q4W: 273, Q8W: 371, PBO: 376 pts), BL characteristics were generally balanced across treatment groups. The mean age was 53 yrs; 55% of pts were male. At BL, mean duration of PsA (7.3 yrs), PsA-modified vdH-S total (27.0) and erosion (13.5) scores, and tender (20.7) and swollen (11.9) joint counts indicated established and highly active joint disease. The primary and major secondary endpoints were met. Significantly greater proportions of GUS Q4W (67%) and Q8W (68%) vs PBO (47%) pts achieved ACR20 response at W24 (P< 0.001; Fig 1A). Pts receiving GUS Q4W and Q8W exhibited significantly less radiographic progression vs PBO at W24 (least squares mean changes in PsA-modified vdH-S score of 0.55 and 0.54 vs 1.35; P=0.002 and < 0.001, respectively; Fig 1B). Similar response patterns were seen for changes in joint space narrowing and erosion scores, proportions of pts with no radiographic progression, and additional clinical efficacy outcomes at W24 (Table 1). Through W24, AEs occurred in 38%, 42%, and 37% of pts (most commonly respiratory infections, headache, diarrhea, and psoriatic arthropathy), and serious AEs occurred in 2%, 3%, and 3% of pts, in GUS Q4W, GUS Q8W, and PBO groups, respectively. No new safety signals were identified.
Conclusion: APEX is the first study to show significant inhibition of structural progression with both Q4W and Q8W of GUS, a dual-acting selective IL-23i. The GUS safety profile in these biologic-naïve pts with active PsA is consistent with that previously established for GUS across a broad range of pts with PsA, psoriasis, and/or inflammatory bowel disease.
To cite this abstract in AMA style:
Mease P, Ritchlin C, Coates L, Kollmeier A, Zhou B, Jiang Y, Bensley K, Im K, Batra R, Chakravarty S, Rahman P, Van Der Heijde D. Inhibition of Structural Damage Progression with Guselkumab, a Selective IL-23i, in Participants with Active PsA: Results Through Week 24 of the Phase 3b, Randomized, Double-Blind, Placebo-Controlled APEX Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/inhibition-of-structural-damage-progression-with-guselkumab-a-selective-il-23i-in-participants-with-active-psa-results-through-week-24-of-the-phase-3b-randomized-double-blind-placebo-controlled/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inhibition-of-structural-damage-progression-with-guselkumab-a-selective-il-23i-in-participants-with-active-psa-results-through-week-24-of-the-phase-3b-randomized-double-blind-placebo-controlled/

.jpg)