Session Information
Date: Sunday, November 10, 2019
Title: SLE – Animal Models Poster
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: A major challenge in lupus is the elimination of autoreactive plasma cells, which amplify inflammation at peripheral sites via deposition of autoantibody-self antigen complexes. We previously reported that Selective Inhibitors of Nuclear Export (SINEs), developed to treat malignancies including multiple myeloma, prevented lupus nephritis in lupus prone mice (LPM). SINEs potently reduced spontaneous formation of germinal centers (GC) and generation of autoreactive plasma cells in the spleen of LPM. We hypothesized that SINEs mediate their effect by disrupting NF-kB signaling in stromal cells, compromising migration and positioning of T and B cells, and thus affecting production of GC-derived plasma cells. Furthermore, SINEs may directly target plasma cells in the spleen and bone marrow (BM).
Methods: We isolated nuclear and cytoplasmic fractions from splenic stromal cells, stimulated with TNFain the presence of SINEs, and evaluated sequestration of NF-kB signaling components in the nuclei by western blot. Fold changes in mRNA expression for stromal cell-derived homeostatic chemokines (HC) were calculated with quantitative PCR. Morphometric analysis of area covered by fluorescent signal revealed the
in vivo
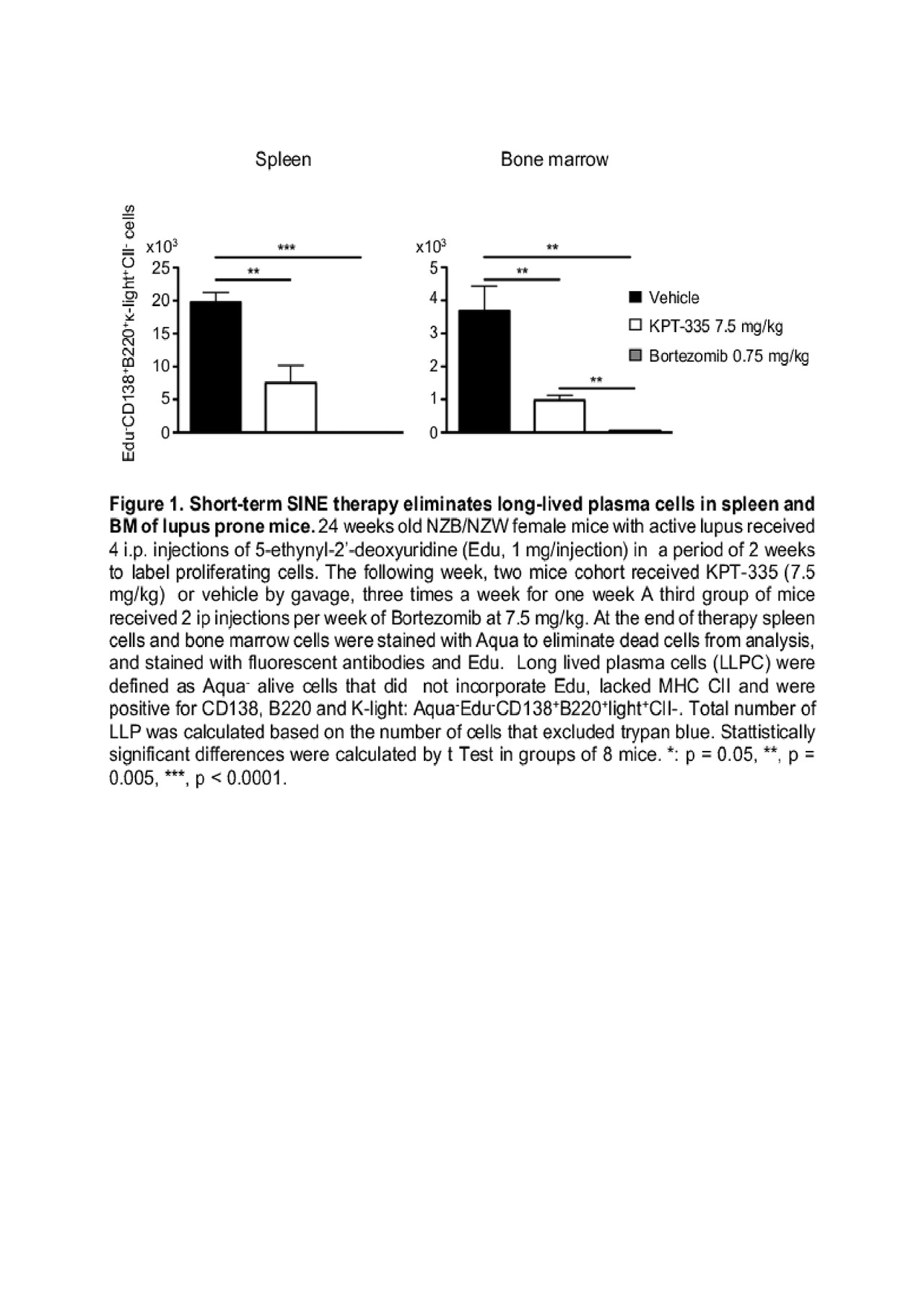
effects of SINEs on splenic HC. Finally, we used flow cytometry to enumerate cycling and non-cycling splenic and BM plasma cells in Edu labelled LPM after short-term therapy with KPT-335 (7.5 mg/kg) or vehicle (n = 8 mice/group).
Results: We demonstrated a significant enrichment for I-kBain the nuclear fraction of stromal cells stimulated with TNFain the presence of SINEs, thus confirming blockade of NF-kB signaling. In addition, fold mRNA expression for CCL19 and CXCL13 – HC that attract CXCR7+T cells and CXCR5+B cells, was significantly reduced in stromal cells incubated with SINEs (Control vs treated, p=0.0028). There was a significant decrease in CXCL13 staining (Control vs treated, p< 0.0001) and the size of follicular dendritic cells networks (Control vs treated, p< 0.0001) in spleens of mice orally treated with KPT-350 at 7.5 mg/kg for 8 weeks (3 administrations/week). As expected, short-term SINE therapy (1 week, 3 administrations/week) caused a two-fold reduction in GC B cells and a significant decrease in T follicular helper cells (Control vs treated, p=0.0043). Of note, short-term SINE therapy caused a global and significant reduction in long-lived plasma cells in spleen (Control vs treated, p=0.004) and BM (Control vs treated, p=0.0071). Unexpectedly, cycling splenic plasmablasts and short-lived plasma cells in the BM were not affected by SINE.
Conclusion: Our data demonstrate that SINEs disrupt NF-kB- dependent production of HC by stromal cells, thus impairing GC formation and compromising autoreactive PC generation. In addition, SINEs have a direct impact on PC survival, including long-lived plasma cells in the BM.
To cite this abstract in AMA style:
Rangel-Moreno J, Garcia-Hernandez M, Owen T, Barnard J, Goldman B, Ritchlin C, Widman D, Gornisiewicz S, Tamir S, Anolik J. Inhibition of Nuclear Pore Export Ameliorates Lupus via Modulation of Plasma Cell Generation and Survival [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/inhibition-of-nuclear-pore-export-ameliorates-lupus-via-modulation-of-plasma-cell-generation-and-survival/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inhibition-of-nuclear-pore-export-ameliorates-lupus-via-modulation-of-plasma-cell-generation-and-survival/