Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Familial Mediterranean Fever (FMF) is characterized by severe systemic and organ inflammation. Successful treatment with rapid remission of symptoms and normalization of laboratory parameters was achieved in most patients with the interleukin-1β inhibitor canakinumab (CAN) in clinical trials. The aim of the present analysis was the evaluation of long-term efficacy and safety of CAN in pediatric (age ≥2 years) and adult patients with FMF with respect to weight-dependent CAN dosing in routine clinical practice.
Methods: RELIANCE is a prospective, non-interventional, observational study based in Germany. Patients with clinically confirmed diagnoses of autoinflammatory periodic fever syndromes routinely receiving CAN are enrolled. Efficacy and safety parameters, CAN dosing as well as weight were recorded at baseline and assessed at 6-monthly intervals within the 3-year observation period of the study.
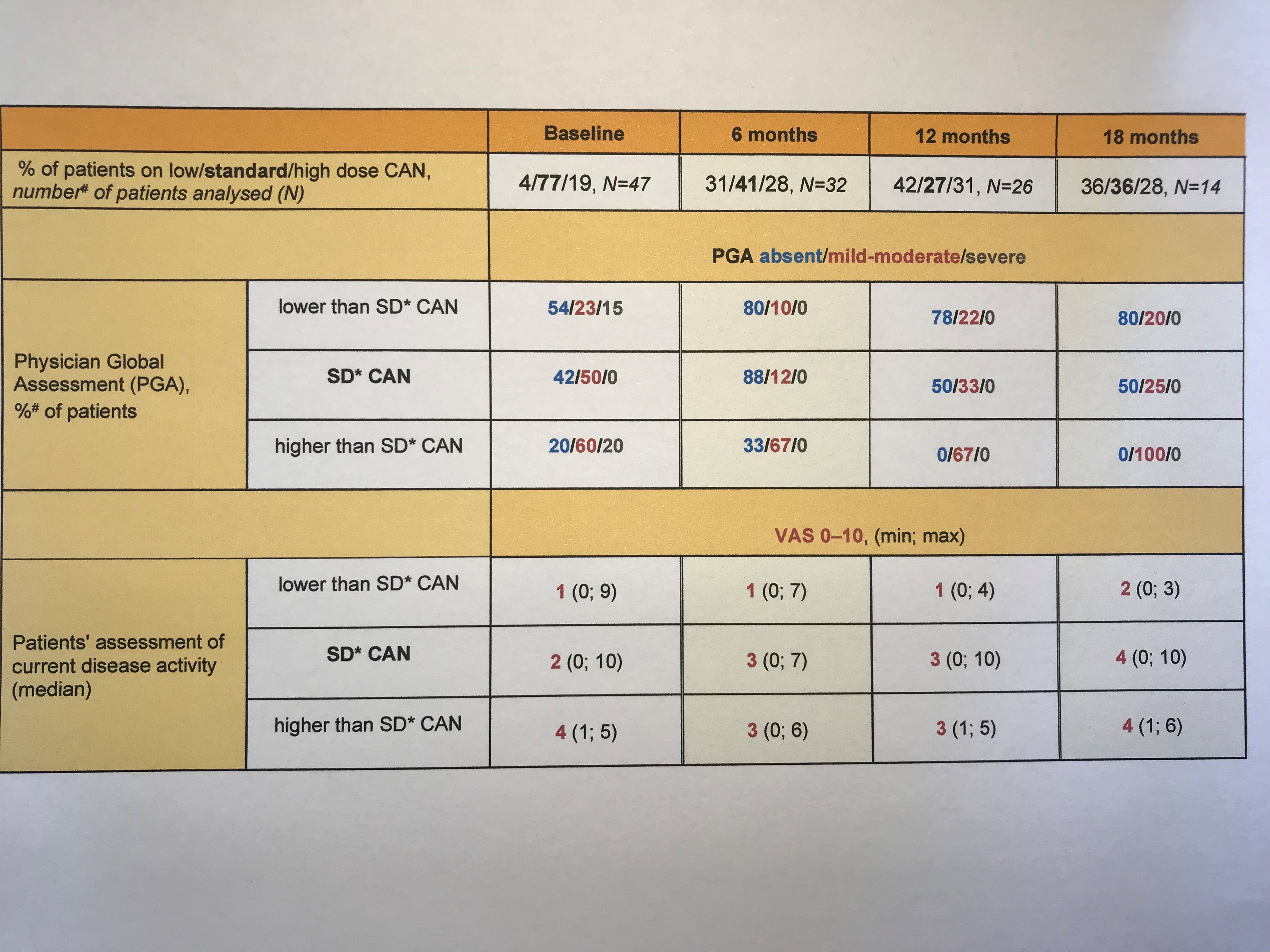
Results: The interim analysis of the RELIANCE Registry comprises data of 54 FMF patients enrolled by December 2020. Of these, the % of patients reported to receive standard dose CAN (SD CAN; 150 mg or 2mg/kg respectively per 4 weeks) halved from 77% at baseline to 36% at month 18 in favor of less than SD CAN (< 87.5% of SD) and higher than SD CAN ( >112.5% of SD). Patients´ and physicians´ rating of disease activity was higher in patients receiving SD CAN and higher (table 1), even though CRP was equally well controlled in all three dosing groups. A total of 11 serious adverse events was reported, of which 1 case of tonsillectomy was classified as drug-related.
Conclusion: The present interim data from the RELIANCE study confirm efficacy and safety of long-term CAN treatment in clinical routine. CRP levels were well controlled in all dosing groups. Remaining disease activity was mainly observed in patients under SD CAN and especially higher than SD CAN.
*Body weight >40 kg: SD is 150 mg per 4 weeks; Body weight ≤40 kg: SD is 2 mg/kg per 4 weeks #Numbers/percentage do not sum up to N=54/100%, due to unknown weight of some patients.
To cite this abstract in AMA style:
Henes J, Blank N, Kallinich T, Dressler F, Horneff G, Foeldvari I, Hufnagel M, Kortus-Goetze B, Weller-Heinemann F, Meier F, Weber-Arden J, Kuemmerle-Deschner J. Influence of Canakinumab Dosing on Efficacy and Safety of Long-term Treatment in Patients with Familial Mediterranean Fever – Interim Analysis of the RELIANCE Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/influence-of-canakinumab-dosing-on-efficacy-and-safety-of-long-term-treatment-in-patients-with-familial-mediterranean-fever-interim-analysis-of-the-reliance-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/influence-of-canakinumab-dosing-on-efficacy-and-safety-of-long-term-treatment-in-patients-with-familial-mediterranean-fever-interim-analysis-of-the-reliance-registry/