Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Infliximab (IFX) has become a mainstay of treatment of many different immune-mediated inflammatory diseases. With the wide range of IFX dosing and dosing intervals, adjustments are often made on clinical parameters without consideration of IFX pharmacokinetics (PK). Measuring clearance (CL) of IFX may be useful for individual dosage adjustment as higher CL is associated with immunogenicity (antibodies to IFX [ATI]), suboptimal exposure (< 8 mg/L at trough) with resultant insufficient neutralization of inflammatory burden. There is paucity of data supporting the notion that optimal PK is achieved in US patients with rheumatic diseases treated with IFX.
Methods: We interrogated a database from a US based clinical PK laboratory offering IFX monitoring and extracted results from patients with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (based on ICD-10 codes beginning with M05, M06 or M08) and having IFX dosing (range 3-10 mg/Kg) and interdose interval provided on test requisition (range 4-8 weeks). Concomitant use of methotrexate was not available. All patient results were de-identified prior to analysis. Serum IFX was measured using a drug tolerant homogenous mobility shift assay. Serum ATI positive status corresponded to titers > 3.1 U/mL. CL, the volume of serum removed each day (expressed as L/day) from drug, was calculated using nonlinear mixed effect models with Bayesian priors using IFX concentrations and ATI status. Minimal effective concentration commensurate with disease control achieved was set at 8 µg/mL, based on prior convention (1). Lower limit of quantification was 1 µg/mL. The impact of lower (< 0.3 L/day), intermediate (0.3-0.8 L/day) and higher clearance ( >0.8 L/day) cutoffs on PK parameters was analyzed using Fisher Exact and logistic regression tests.
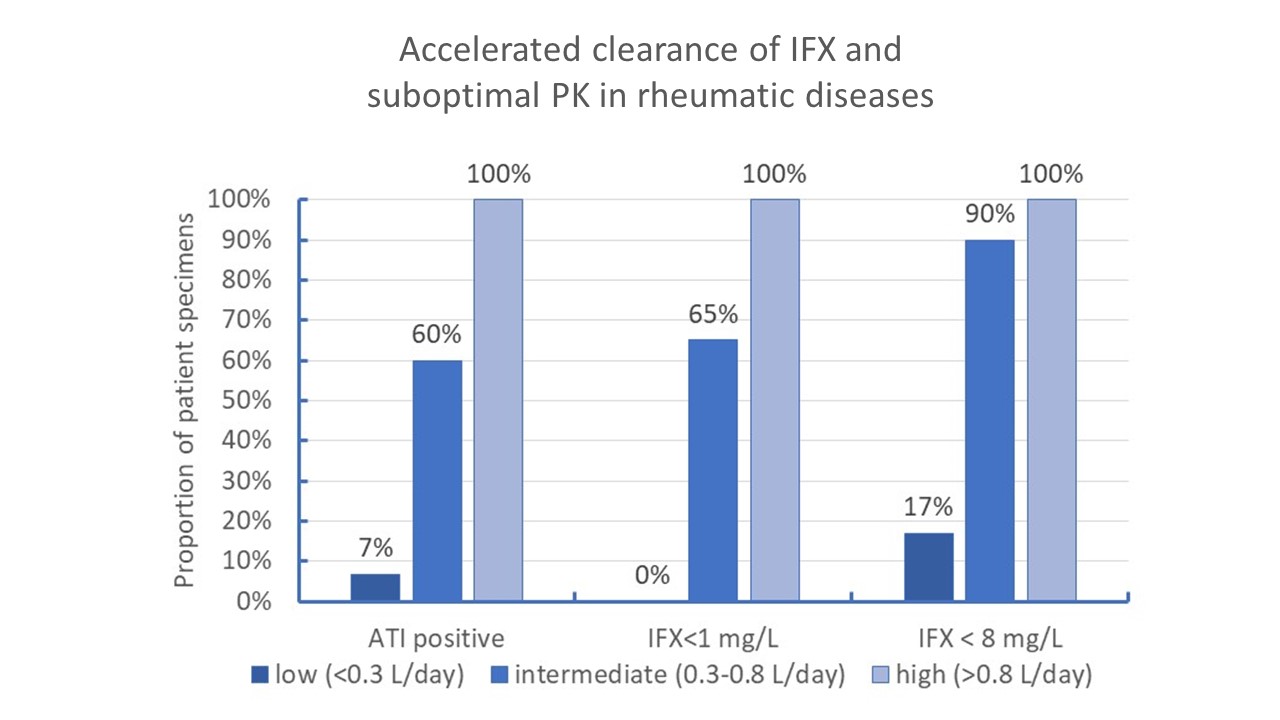
Results: We identified a total of 161 patient specimens ordered by 74 practices and submitted for testing (73% female, mean age =57 [Interquartile range, IQR: 37-69 years]). Median dose was 7.5 mg/Kg normalized to every 8 weeks (IQR: 5-13 mg/Kg every 8 weeks).Median IFX level was 14 µg/mL (IQR: 2.8-28.9 µg/mL), suboptimal exposure (< 8µg/mL) was observed in 36% patients, 22% presented with ATI, 18% had IFX concentration < 1µg/mL. Median CL was 0.231 L/day [IQR: 0.173-0.312 L/day]. Higher and intermediate CL were observed in 2% [3/161] and 25% ([40/161] patient specimens, respectively). Inadequate exposure was observed in most patient specimens having intermediate or high CL and there was little immunogenicity (7%) in the presence of lower CL. In the presence of intermediate or higher CL, there was 47.8 (95%CI: 15.2-150.1) and 23.2 (95%CI: 8.9-60.3) fold higher likelihood of having concentration below 8 mg/L and immunogenicity, respectively (p< 0.001).
Conclusion: This preliminary data suggests that IFX dosing may be suboptimal in a significant proportion of patients with RA and other inflammatory rheumatic diseases. Dose optimization (achieving IFX levels >8 mg/L at trough) might benefit patients who demonstrate inadequate disease control, particularly those who manifest accelerated CL, in part due to immunogenicity.
(1) Mulleman D., et al. Arthritis Res Ther. 2009;11:R178. doi: 10.1186/ar2867
To cite this abstract in AMA style:
Rosenstein E, Weinblatt M, Everts-van der Wind A, Conklin J, Dervieux T. Infliximab Clearance a Predictive Factor of Pharmacokinetic Origin in Relation to Suboptimal Pharmacokinetics and Immunogenicity in United States Based Rheumatology Practices [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/infliximab-clearance-a-predictive-factor-of-pharmacokinetic-origin-in-relation-to-suboptimal-pharmacokinetics-and-immunogenicity-in-united-states-based-rheumatology-practices/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/infliximab-clearance-a-predictive-factor-of-pharmacokinetic-origin-in-relation-to-suboptimal-pharmacokinetics-and-immunogenicity-in-united-states-based-rheumatology-practices/