Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Ixekizumab (IXE) is a high-affinity monoclonal antibody that selectively targets IL-17A. IL-17 inhibitors have shown efficacy for radiographic axial spondyloarthritis (r-axSpA), with IXE currently under study. As biologics may be associated with increased infections, we report on infections in patients with active r-axSpA treated with IXE.
Methods: Eligible patients met ASAS axSpA criteria, had radiographic sacroiliitis according to mNY criteria, and were either biologic-naïve (COAST-V: NCT02696785) or biologic-experienced (COAST-W: NCT02696798). Patients randomized to receive 16 weeks of double-blinded (DB) subcutaneous (SC) IXE 80 mg (once every 4 weeks [Q4W] or 2 weeks [Q2W]), placebo (PBO), or adalimumab (ADA, active reference) 40 mg Q2W (COAST-V only). All patients initially randomized to PBO or ADA (COAST-V) rerandomized to IXE at Weeks 16 through 52. The number and percentage of patients with infections are summarized for DB (Weeks 0 to 16), extended (Weeks 16 to 52), and combined treatment periods (Weeks 0 to 52).
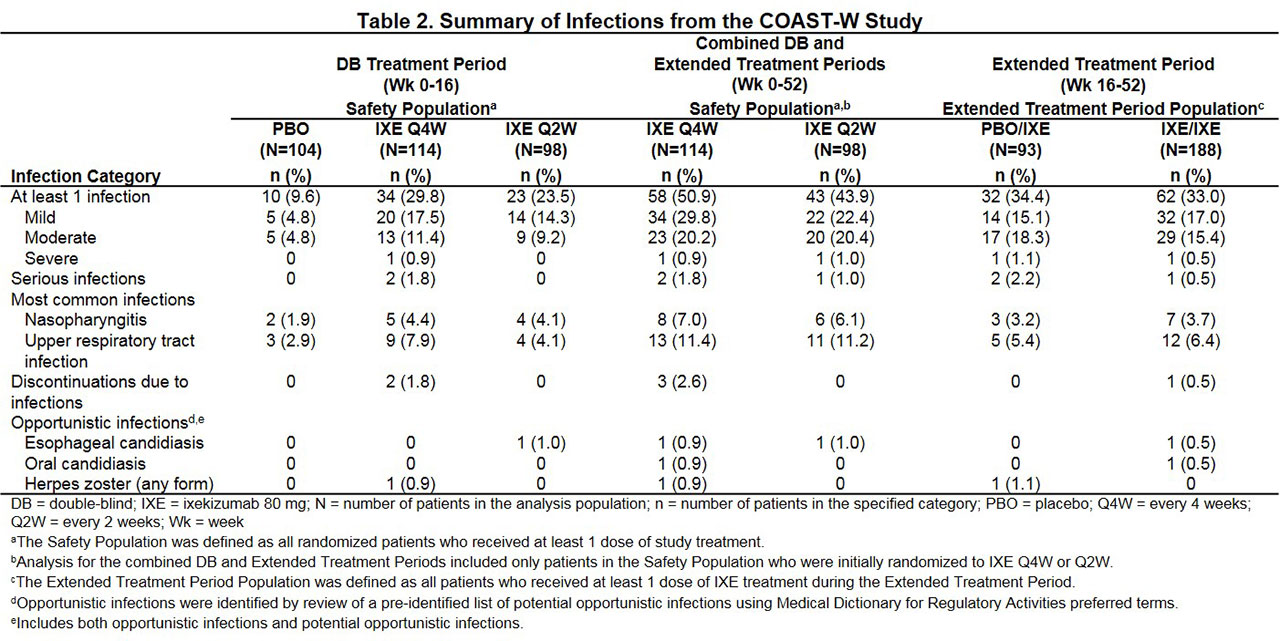
Results: Total patients randomized were 341 in COAST-V (Table 1) and 316 in COAST-W (Table 2).
In COAST-V through Week 16, infections occurred in 19.8% (16/81), 20.5% (17/83), and 15.1% (13/86) of IXE Q4W, IXE Q2W, and PBO patients, respectively, and through Week 52 in 37.0% (30/81) and 43.4% (36/83) of patients initially randomized to IXE Q4W and Q2W, respectively. In COAST-W through Week 16, infections occurred in 29.8% (34/114), 23.5% (23/98), and 9.6% (10/104) of IXE Q4W, IXE Q2W, and PBO patients, respectively, and through Week 52 in 50.9% (58/114) and 43.9% (43/98) of patients initially randomized to IXE Q4W and Q2W, respectively.
In both studies, serious infections were reported in < 2% of IXE patients through Week 16 and in < 3% of patients during extended treatment. Most infections were mild or moderate in severity, and the most common infections were upper respiratory tract infection and nasopharyngitis. Overall, < 3% of patients discontinued due to infection, and no deaths due to infections occurred in either study.
In COAST-V, no IXE patients reported opportunistic infections through Week 16, and < 3% of patients reported opportunistic infections during extended treatment (esophageal candidiasis, fungal esophagitis, herpes simplex, herpes zoster [unidermatomal]). In COAST-W, ≤1% of IXE patients reported opportunistic infections through Week 16 (esophageal and oral candidiasis, herpes zoster), and < 2% of patients reported opportunistic infections during extended treatment (esophageal and oral candidiasis, herpes zoster). Opportunistic infections were mild or moderate in severity and none were serious. No tuberculosis cases were reported in either study and most candida and herpes infections resolved without recurrence.
Conclusion: Infection frequencies in COAST-V and COAST-W over 52 weeks are consistent with those in previous IXE PsA and PsO trials. Infection frequencies were similar between the IXE doses and study periods, with minimal difference between studies; thus, previous exposure to biologics did not affect the incidence of infections. Incidences of serious infections, opportunistic infections, and discontinuations due to infections were low in both studies.
To cite this abstract in AMA style:
Magrey M, Navarro-Compán V, Garces S, Baraliakos X, Sandoval D, Lisse J, Santisteban S, Adams D, Zhao F, Inman R. Infections in Patients with Active Radiographic Axial Spondyloarthritis Treated with Ixekizumab in 2 Phase 3 Clinical Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/infections-in-patients-with-active-radiographic-axial-spondyloarthritis-treated-with-ixekizumab-in-2-phase-3-clinical-trials/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/infections-in-patients-with-active-radiographic-axial-spondyloarthritis-treated-with-ixekizumab-in-2-phase-3-clinical-trials/