Session Information
Date: Monday, October 22, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster II: Clinical/Epidemiology Studies
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: PsA is a chronic inflammatory disease associated with psoriasis. For optimal quality of life (QoL) improvements, all PsA symptoms should be managed. We examine the association between improvements in joint or skin-related symptoms and QoL in patients (pts) with active PsA.
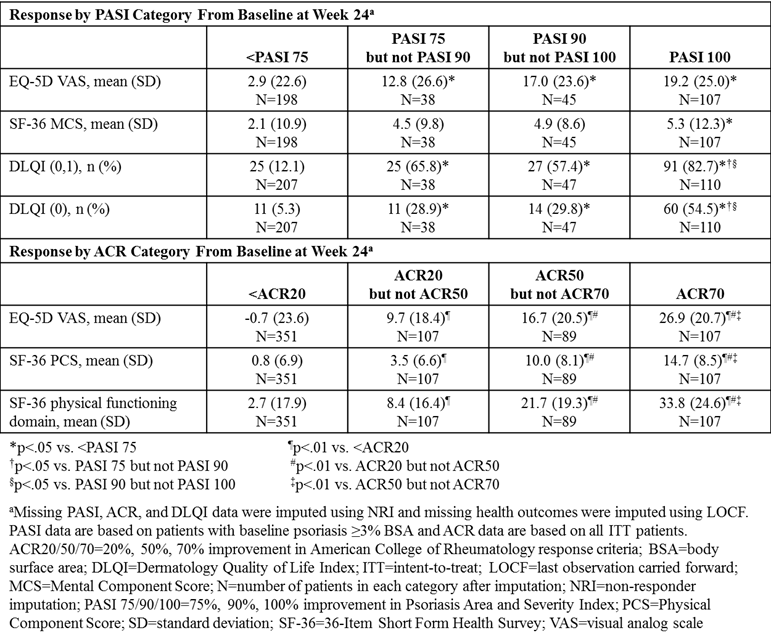
Methods: Data from the 24-week (wk), randomized, double-blind, placebo-controlled periods of 2 Phase 3 clinical trials (SPIRIT-P1, NCT01695239; SPIRIT-P2, NCT02349295) were combined to assess pts with active PsA who were biologic-naïve (N=316) or were intolerant to TNF inhibitors (N=363) and received sc placebo or 80 mg ixekizumab every 2 or 4 wks, after a 160 mg starting dose.1,2 All pts met the Classification Criteria for PsA. QoL outcomes were assessed in categories of pts based on Psoriasis Area and Severity Index (PASI) or ACR responses at Wk 24. ACR response categories included pts not achieving ACR20, pts achieving ACR20 and <ACR50, ACR50 and <ACR70, and ACR70. PASI response categories included pts with baseline (BL) psoriasis ≥3% body surface area not achieving PASI 75, pts achieving PASI 75 and <PASI 90, PASI 90 and <PASI 100, and PASI 100. The percentage of pts achieving Dermatology Quality of Life Index (DLQI) score 0-1 or 0, indicating no impact on QoL, and mean change in EQ-5D visual analog scale (VAS) and 36-Item Short Form Health Survey (SF-36) Mental Component Score (MCS) was evaluated across PASI categories at Wk 24. Mean change in EQ-5D VAS, SF-36 Physical Component Score (PCS), and SF-36 physical functioning was evaluated across ACR categories at Wk 24. Missing data were imputed using non-responder imputation for categorical data and last observation carried forward for continuous data. Response categories were compared using logistic model for categorical outcomes; analysis of covariance was used for continuous outcomes, with BL health outcome score as a covariate.
Results: At Wk 24, the greatest improvements in QoL as assessed by EQ-5D were associated with achieving PASI 100 or ACR70. Also, the proportion of pts achieving DLQI score 0-1 or 0 and the mean change in SF-36 MCS increased with PASI improvement, with the greatest QoL improvements in pts achieving PASI 100. Similarly, from BL-Wk 24, SF-36 PCS and SF-36 physical functioning improved with ACR response, with the greatest improvements alongside ACR50 and ACR70 responses.
Conclusion: Incremental QoL benefits were observed across PASI and ACR response categories. Clear skin (PASI 100) was associated with the greatest improvements in skin-specific QoL, mental aspects, and general QoL. Pts achieving ACR50 and ACR70 achieved the greatest improvements in physical aspects and general QoL. These data provide more evidence that optimal QoL improvements are associated with greater improvements in joint and skin-related PsA symptoms.
References:
1. Mease PJ et al. Ann Rheum Dis 2017;76:79-87
2. Nash P et al. Lancet 2017;389:2317-27
To cite this abstract in AMA style:
Smolen JS, Shrom D, Lin CY, Birt J, Schett G, Gottlieb AB. Incremental Benefits to Quality of Life Associated with Achieving Higher Levels of American College of Rheumatology Response and Skin Clearance in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/incremental-benefits-to-quality-of-life-associated-with-achieving-higher-levels-of-american-college-of-rheumatology-response-and-skin-clearance-in-patients-with-psoriatic-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incremental-benefits-to-quality-of-life-associated-with-achieving-higher-levels-of-american-college-of-rheumatology-response-and-skin-clearance-in-patients-with-psoriatic-arthritis/