Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) treatments include steroids, antimalarials, immunosuppressants and/or biologics, though the use of biologics has been reported as minimal in claim-based studies examining real-world treatment patterns between 2006 to 2016. Here, we examine choice of treatments in the last three years within community rheumatology practices to understand current use of biologics. In addition, we examine persistence to belimumab, the most commonly used biologic for SLE, and association with different treatment and patient characteristics.
Methods: The ARN-TRIO Rheumatology registry contains EMR (fielded and open text), lab, procedure, infusion, medical claims, and specialty pharmacy data generated in care of >75,000 patients by ARN, a network of independent practices with >200 rheumatologists across the US. Data for adult (18+) patients with SLE who initiated or switched to a new treatment (starts) from Jan 2017 to May 2020 were included in this study. Comparisons between groups were made using t-test for continuous variables and chi-square or Fischer’s exact tests for categorical variables. Time to events analyses were conducted by Kaplan-Meier and subsequent log-rank test.
Results: A total of 5,797 SLE patients started 10,913 distinct treatments in the observation window. Comparing starts by drug class between 2017 to 2019 indicated annualized growth rates of 20% for biologics, 7% for immunosuppressants, 2% for steroids, and -2% for antimalarials, with significant differences (p< 0.050) between 2019 and 2017 start percentages for all drug classes except steroids. [FIGURE 1] To compare populations receiving different drug classes, patients were classified into biologic or non-biologic groups based on initial treatment (index). [TABLE 1] At index, 91% (5,272) received non-biologic therapy; 67% (3,884) received antimalarials, 36% (2,098) steroids, and 22% (1,287) immunosuppressants. Patients treated with biologic therapies at index mostly received belimumab (66%, 347/525); rituximab was used for 11% (60/525) of patients. Compared to the non-biologic group, the biologics group differed in use of other drug classes and payer, but not in age, gender, or race. For patients receiving belimumab, the mean time to discontinuation was 838 days (25th percentile = 620 days, median not reached). Time to belimumab discontinuation differed by concomitant use of steroids, antimalarials, or immunosuppressants and these individual associations persisted regardless of the presence of other drug classes. [FIGURE 2] Time to belimumab discontinuation was not associated with age, gender, race, or payer.
Conclusion: The use of biologics has increased considerably since 2017 though these agents are still limited to a fifth of treatment starts. Aside from a slight but significantly higher percentage with commercial coverage, the group initiating biologics was similar to the non-biologic group for patient characteristics. For patients that received belimumab, persistence on therapy was considerable and increased in populations receiving concomitant steroids, antimalarials, or immunosuppressants.
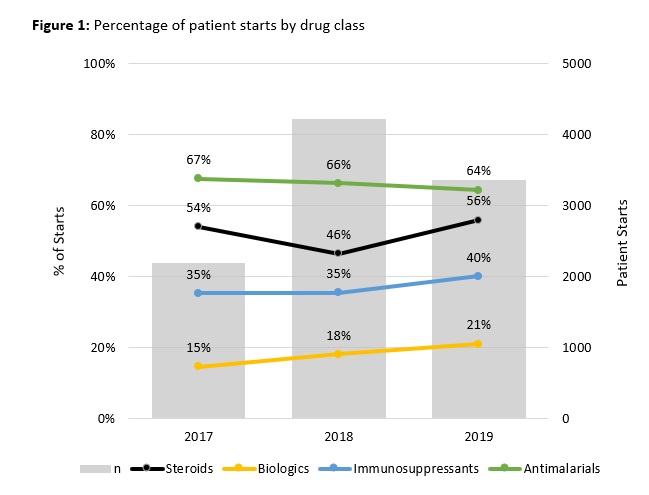 Figure 1: Percentage of patient starts by drug class
Figure 1: Percentage of patient starts by drug class
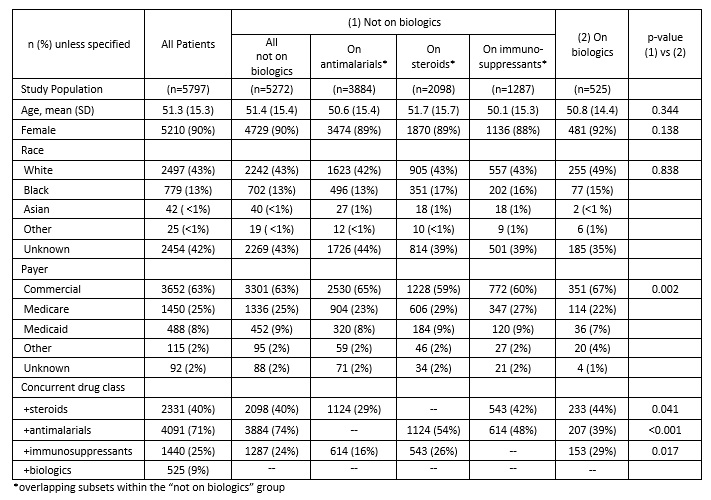 Table 1: Patient Characteristics at index
Table 1: Patient Characteristics at index
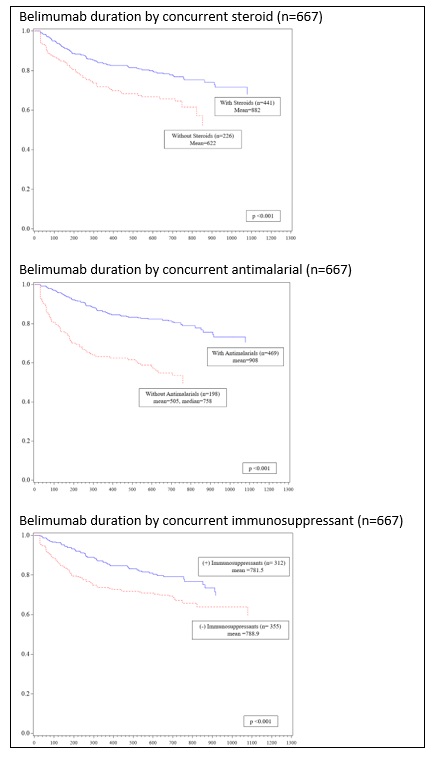 Figure 2: Days to belimumab discontinuation by concurrent drug class
Figure 2: Days to belimumab discontinuation by concurrent drug class
To cite this abstract in AMA style:
Helfgott S, Broestl J, Huston K, Rane D, Singh J, Soloman N, Edgerton C. Increasing Use of Biologics in Treatment of Systemic Lupus Erythematosus Patients in US Clinical Practice: Real-World Observations from Trio Health and the American Rheumatology Network [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/increasing-use-of-biologics-in-treatment-of-systemic-lupus-erythematosus-patients-in-us-clinical-practice-real-world-observations-from-trio-health-and-the-american-rheumatology-network/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increasing-use-of-biologics-in-treatment-of-systemic-lupus-erythematosus-patients-in-us-clinical-practice-real-world-observations-from-trio-health-and-the-american-rheumatology-network/
