Session Information
Date: Sunday, November 10, 2019
Title: Measures Of Healthcare Quality Poster I: Testing, Screening, & Treating
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rituximab (RTX) is associated with a large cost burden on the healthcare system and treatment delays that often exceed one month because the long infusion duration ( >4 hours) limits daily capacity. Time-drive activity-based costing (TDABC) is a novel method to estimate costs that can identify opportunities for economic and process efficiencies. The TDABC approach examines actual time spent with each resource and its per-unit cost. We performed TDABC of RTX administration in rheumatology to identify key cost drivers, project potential cost- and time-saving opportunities, and enable future cost-effectiveness research.
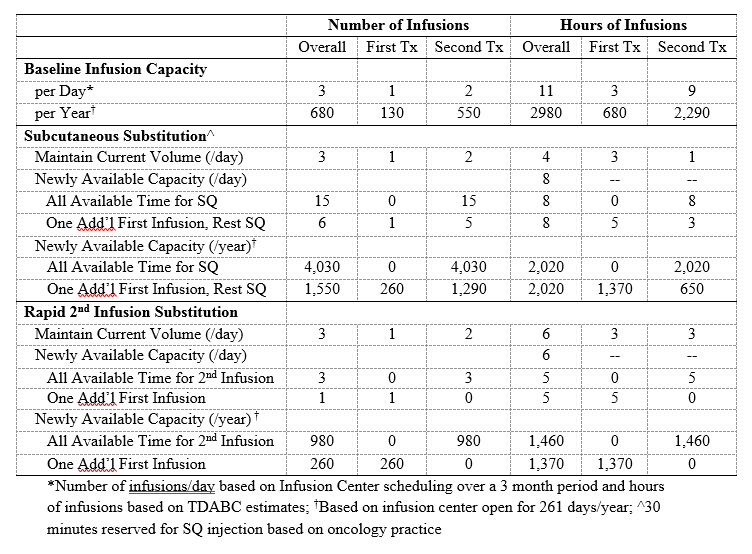
Methods: We implemented TDABC throughout the care pathway (e.g., orders, pharmacy preparation, scheduling, administration) for 26 patients receiving a total of 30 RTX infusions in an academic hospital-based infusion center (IC). The base-case was one treatment cycle which included two 1,000mg RTX infusions administered using standard rates over two visits. We assumed a 23% rebate on drug costs. After collecting base-case data on provider timing, pre-treatment regimens, and infusion rates, we then varied these in sensitivity analyses paying particular attention to varying (1) the drug (biosimilar vs originator), and (2) administration, including the use of subcutaneous (SQ) or rapid (e.g., 90-minute) infusion protocols. We estimated the mean number of RTX infusions per day using 3 months of IC scheduling data and associated visit length (hours) using estimates from TDABC. We then assessed the potential effect of two alternative strategies on annual capacity: a) SQ RTX and b) rapid 2nd infusion protocols. We assumed that SQ RTX would require a 30-minute appointment based on oncology practice.
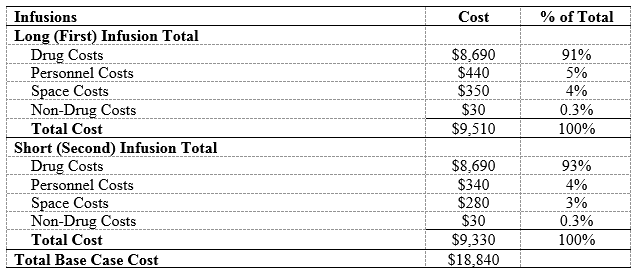
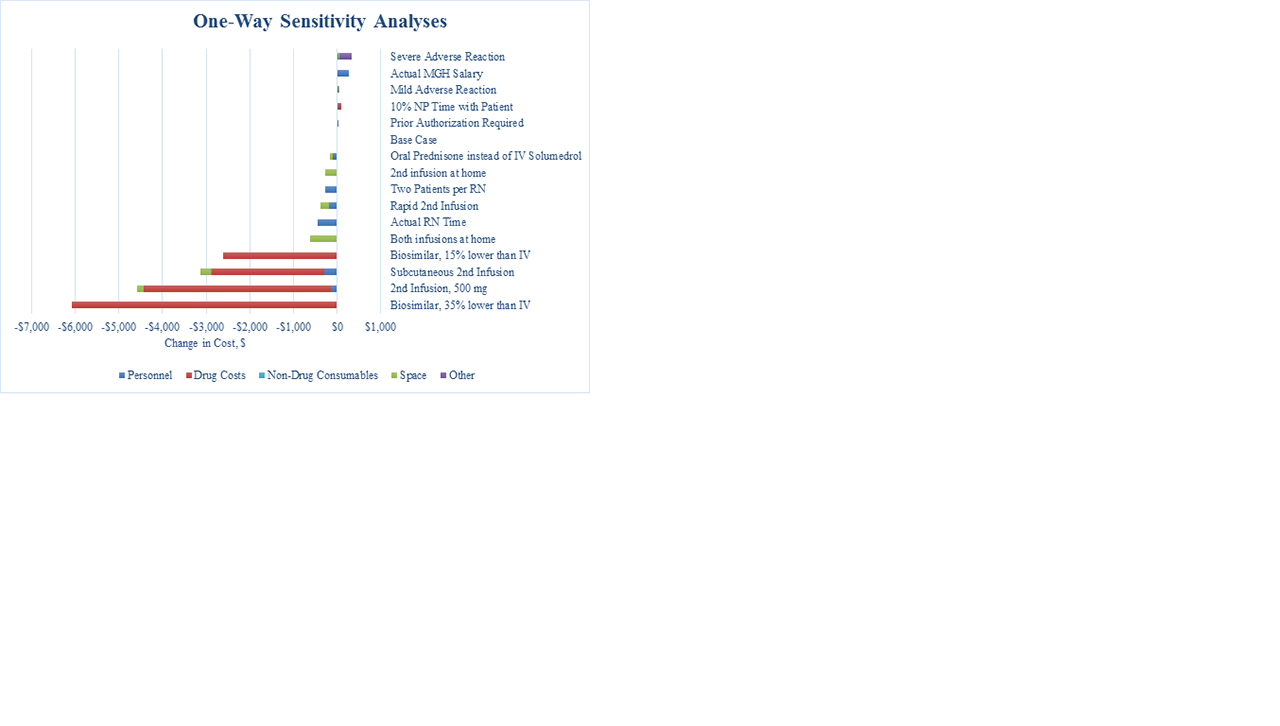
Results: In the base-case, RTX administration costs $18,840 (Table 1), 92% ($17,380) from drug costs; the remaining costs were due to personnel ($780, 4%), non-drug consumables ($60, 0.3%), and space ($630, 3%). In one-way sensitivity analyses (Figure), the greatest cost savings were associated with biosimilar RTX at a 35% cost reduction (-$6,080) and substitution of the 2nd dose with SQ RTX (-$3,170). SQ RTX use led to savings on personnel (-$290, -37%), consumable (-$2,640, -15%), and space (-$250, -39%) costs. In a “best case” scenario, when we used IV biosimilar RTX for the 1st dose, SQ RTX for the 2nd dose, assigned two patients per RN, and used oral prednisone (rather than IV solumedrol) as pre-treatment for the 1st dose, total savings reached -$6,350 (-34%). Using SQ RTX would increase the number of RTX treatments available by up to 15 patients per day, a 6-fold increase in capacity (Table 2). Using a rapid 2nd infusion would increase the number of RTX treatments available in one day by up to 3, an increase in capacity of 2-fold (Table 2).
Conclusion: Using a novel costing method, we found that drug price is the primary driver of the cost of RTX treatment and that varying non-drug factors has minimal impact on overall costs. The ultimate list price of biosimilar RTX may substantially impact the cost of RTX administration on the healthcare system. However, use of biosimilar and SQ RTX may reduce costs by 34% and appreciably increase administration capacity, thereby reducing delays in treatment.
To cite this abstract in AMA style:
Wallace Z, Harkness T, Blumenthal K, Choi H, Stone J, Walensky R. Increasing Capacity and Reducing Costs of Rituximab Administration [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/increasing-capacity-and-reducing-costs-of-rituximab-administration/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increasing-capacity-and-reducing-costs-of-rituximab-administration/