Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Women of child-bearing age with underlying rheumatic disease such as systemic lupus erythematous (SLE) with positive anti-phospholipid antibodies (aPL) are at an increased risk of thrombosis. American College of Rheumatology (ACR) strongly recommends against the use of combined estrogen-progestin contraceptives in women with rheumatologic disease with positive aPL due to increased risk of thromboembolism. There is a strong recommendation for intrauterine devices (IUD) or the progestin-only pill in this population. The depo-medroxyprogesterone acetate intramuscular shot is not recommended due to limited data based on non-rheumatological disease patients suggesting that it results in higher thrombotic risk. The progestin implant is not recommended due to insufficient data. We investigated rates of thrombotic events in SLE patients with positive aPL on various forms of contraception.
Methods: Female patients age >18 years from January 2012- December 2021 with SLE and aPL testing were included in this study. These patients were divided into those with at least one positive aPL test and those with negative testing. The group with positive aPL testing was sub-categorized into those receiving contraception; this group was further sub-categorized into those receiving estrogen-based contraception (defined as oral combined estrogen-progestin pills) and those receiving progestin-based contraception (defined as levonorgestrel IUD, progestin only pill, depo-medroxyprogesterone acetate intramuscular shot or progestin implant). Lastly, the group receiving progestin-based contraception was divided into the specific form of progestin-contraception used and these groups were compared to one another. The primary end point in all groups was incidence of a thrombotic event defined as a cerebrovascular accident (CVA), pulmonary embolus (PE), or deep venous thrombosis (DVT). Statistical analysis was based on two-tailed chi-square test using GraphPad software. A p value < 0.05 was considered significant.
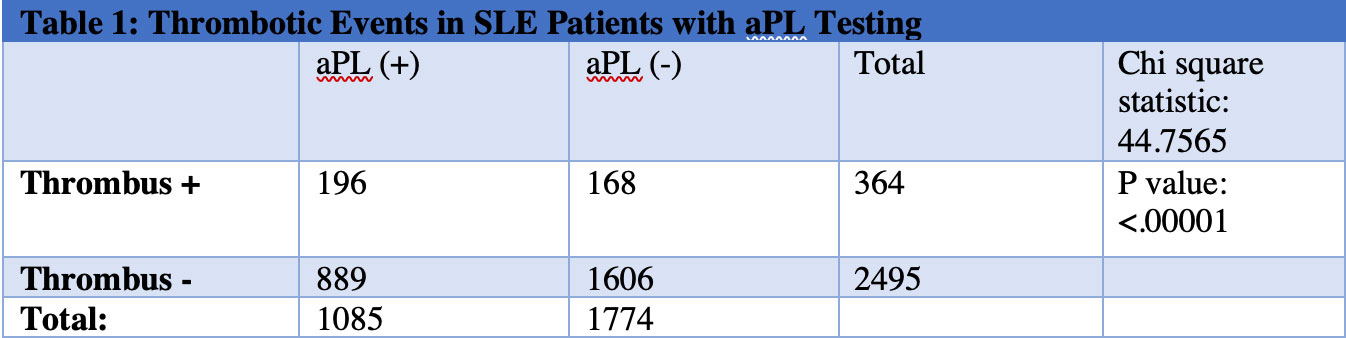
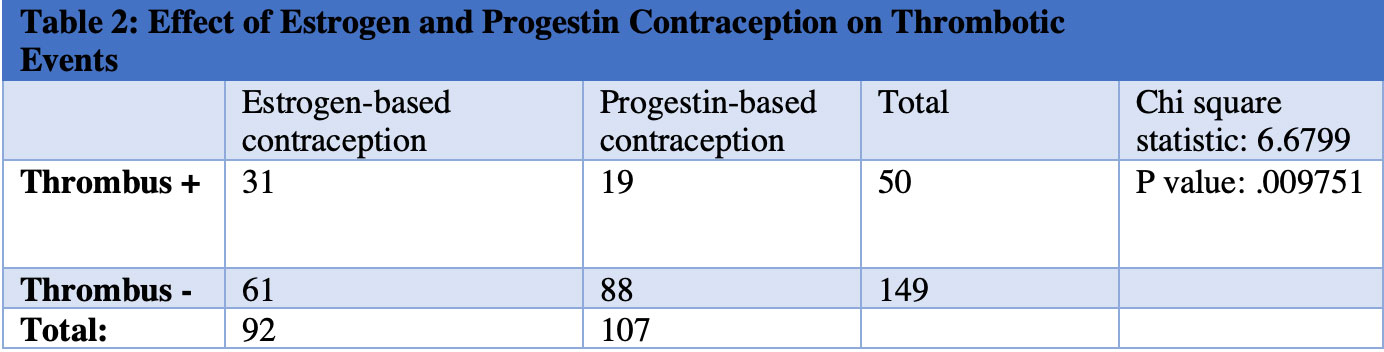
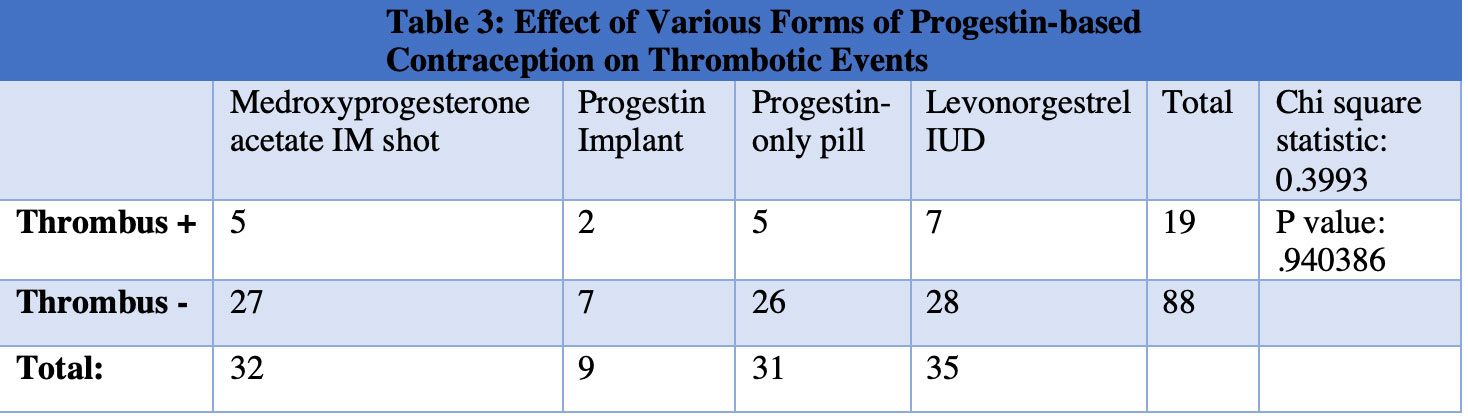
Results: SLE patients with positive aPL had a statistically significant increase in thrombotic events (Table 1) and those on estrogen-based contraception had a statistically significant increase in thrombotic events compared to those receiving progestin-based contraception (Table 2). Next, each form of progestin-based contraception was compared to one another and there was no statistically significant difference in thrombotic events between groups (Table 3).
Conclusion: Combined estrogen-progestin contraception in SLE patients with positive aPL conferred an increased risk of thrombosis relative to progestin-only contraception. We hereby identify an at-risk category of SLE patients with positive aPL on estrogen-based contraception, which is an area for improvement in our patient population. ACR recommends the use of an IUD or the progestin-only pill for SLE patients with positive aPL and does not currently recommend depo-medroxyprogesterone acetate IM shot or progestin implant. Our findings suggest that all forms of progestin-based contraception may be safe in SLE patients with positive aPL.
To cite this abstract in AMA style:
Bilal H, Baluch A, Perl A. Increased Prevalence of Thrombotic Events in Anti-Phospholipid Antibody-Positive SLE Patients on Estrogen-Containing Contraception [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/increased-prevalence-of-thrombotic-events-in-anti-phospholipid-antibody-positive-sle-patients-on-estrogen-containing-contraception/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increased-prevalence-of-thrombotic-events-in-anti-phospholipid-antibody-positive-sle-patients-on-estrogen-containing-contraception/