Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Generated under oxidative stress, malondialdehyde-acetaldehyde adducts (MAA), and antibody responses to MAA appear to facilitate loss of immune tolerance and generate pro-inflammatory responses in rheumatoid arthritis (RA). Both MAA adduct formation and anti-MAA antibody levels are enriched in joint tissues of RA patients compared to those with non-inflammatory arthritis, and MAA appears to co-localize with citrullination in joint tissue. Because oxidative stress and autoimmunity are also key components of extra-articular complications of RA, including interstitial lung disease (ILD), we assessed MAA and anti-MAA antibody responses in RA-ILD.
Methods: Using banked serum from the Veterans Affairs Rheumatoid Arthritis (VARA) registry, we assessed serum anti-MAA antibody concentrations (IgA, IgM, and IgG via ELISA) in RA patients with and without diagnosis codes for ILD. Log-transformed anti-MAA antibody concentrations were compared by group using t-tests and linear regression models adjusted for age, sex, smoking status, disease activity, and anti-CCP antibody positivity. Additionally, we analyzed lung tissue sections from subjects with RA-ILD, non-RA ILD, emphysema, and controls (n=2 per group) available through the NIH’s Lung Tissue Resource Consortium. Sections were stained using a MAA-specific rabbit polyclonal antibody and a citrulline-specific mouse IgM monoclonal antibody, and imaged with a confocal laser scanning microscope.
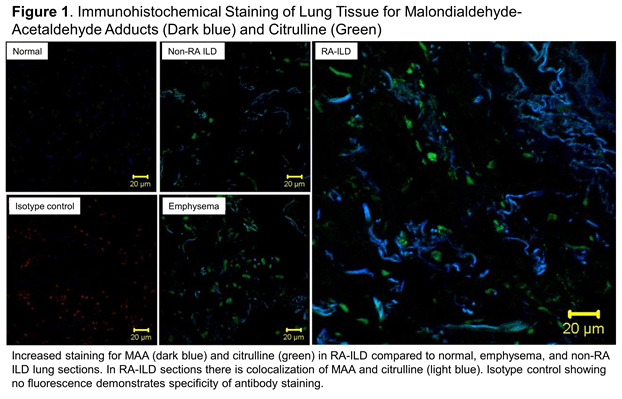
Results: In a male predominant (90%), established RA patient cohort (mean duration 11 years) with frequent smoking history (78%), 103 subjects had a diagnosis code for ILD. Serum concentrations of IgA and IgM anti-MAA antibody were significantly higher in RA-ILD subjects, even after adjustment for anti-CCP antibody positivity, disease activity, and smoking status (Table 1). IgG anti-MAA antibody concentrations were also marginally higher in RA-ILD subjects, but were not statistically significant. Lung tissue demonstrated staining for MAA in non-RA ILD and emphysema; however, qualitatively, staining was highest in RA-ILD (Figure 1). In addition, staining demonstrated that MAA and citrulline co-localized in lung tissue from RA-ILD.
Conclusion: Anti-MAA antibodies, IgA and IgM, are higher in RA patients with a diagnosis of RA-ILD. Along with the enhanced staining of MAA-modified proteins in lung tissue samples from RA-ILD, our findings suggest that MAA adduct formation and resulting adaptive immune responses could play an important role in the pathogenesis of RA-ILD and also serve as informative disease biomarkers.
|
Table 1. Associations of RA-ILD with serum anti-MAA antibody expression. |
|||
|
|
RA-ILD N=103 |
RA, no lung disease N=1468 |
P value |
|
IgA anti-MAA± |
|
|
|
|
Concentration |
6.96 (1.07) |
6.40 (1.42) |
<0.001 |
|
β coefficient |
0.503 (0.224, 0.782) |
Referent |
<0.001 |
|
IgM anti-MAA± |
|
|
|
|
Concentration |
8.18 (1.40) |
7.41 (2.00) |
0.002 |
|
β coefficient |
0.718 (0.324, 1.112) |
Referent |
<0.001 |
|
IgG anti-MAA± |
|
|
|
|
Concentration |
7.59 (1.15) |
7.33 (1.45) |
0.08 |
|
β coefficient |
0.232 (-0.056, 0.521) |
Referent |
0.11 |
|
± log transformed Concentrations mean (SD) Regression models with RA-ILD predicting serum anti-MAA antibody titer; adjusted for age, sex, smoking status, anti-CCP positivity, DAS28. |
|||
To cite this abstract in AMA style:
England BR, Thiele GM, Duryee MJ, Ascherman DP, Caplan L, Demoruelle MK, Deane KD, Mikuls TR. Increased Expression of Malondialdehyde-Acetaldehyde Adducts (MAA) and Anti-Maa Antibody in Rheumatoid Arthritis-Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/increased-expression-of-malondialdehyde-acetaldehyde-adducts-maa-and-anti-maa-antibody-in-rheumatoid-arthritis-interstitial-lung-disease/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increased-expression-of-malondialdehyde-acetaldehyde-adducts-maa-and-anti-maa-antibody-in-rheumatoid-arthritis-interstitial-lung-disease/