Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: to evaluate whether TSH increases during febuxostat treatment and factors that could be implicated.
Methods: before starting febuxostat, patients had analysis at baseline and month 3 and 6 including serum urate, estimated glomerular filtration, liver function test, and TSH at baseline and 6-month visit. General characteristics and previous urate-lowering drugs, and concomitant diseases were registered. Patients with altered TSH at baseline were included if T4 was within normal range. All patients showing TSH out of the normal range (0.5-5.0 mU/ml) had to undergo T4 determination while on febuxostat and febuxostat was to be withdrawn if T4 was found not within normal limits. Results are shown as median and interquartile range. Febuxostat was started 80 mg eod for the first month and then all patients escalated to 80 mg/d until the 3-month visit. Febuxostat was then regulated to 80 mg eod for patients showing Sur <3 mg/dl and non-tophaceous gout and to 120 mg/d in patients with serum urate > 6 or > 5 and tophaceous gout.
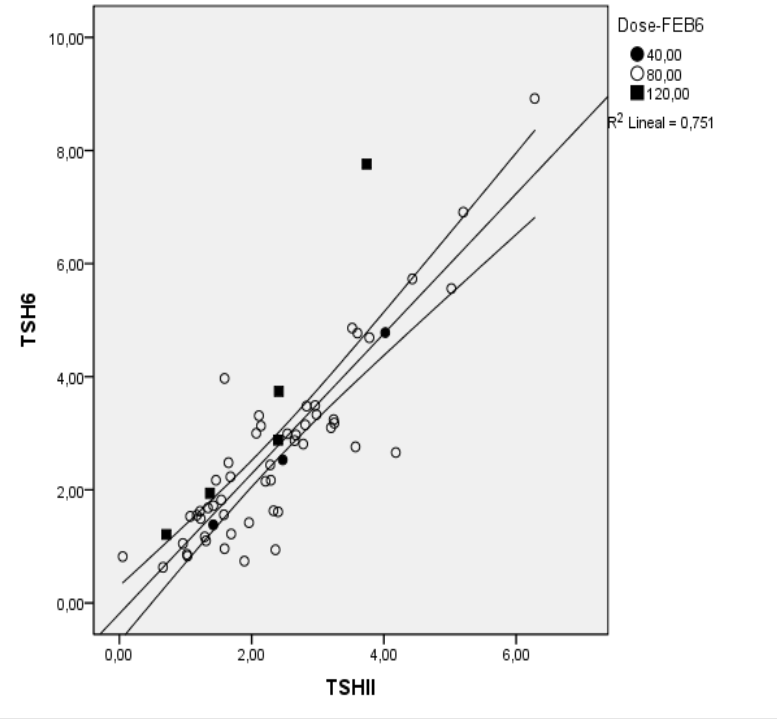
Results: sixty-eight patients were included, mean age 62 years (51-73), time from onset 8 years (2-15), 59% with tophi present, serum urate 9.5 mg/dl (8.7-10.4) glomerular filtration 78 ml/min (52-101), TSH 2.34 U/ml (1.39-2.89). Three (4.4%) patients showed baseline TSH > UNL, but T4 was within normal range. At 6-month 5, 3 patients were on 80 mg eod, 5 on 120 mg/d, and 60 on 60 mg/day, 62/68 (91%) showing serum urate < 6 mg/dl. TSH increased from 2.34 to 2.71(1.51-3.27, p<0.001), 5 (7.3%) showing TSH > UNL ranging from 5.56 to 8.92. None of these patients showed altered T4 levels or any symptom related to hypothyroidsm. There was a close correlation between baseline and final TSH (r2=0.75) (Figure 1). Multivariant analysis showed a that baseline TSH and the dose of febuxostat at 6-month visit were independently associated with TSH levels at 6-month visit (model R2=0.855 ), and with the change in TSH from baseline to 6-month (model R2= 0.396). No patient with baseline TSH < 3 showed TSH above UNL.
Figure 1. Correlation between baseline and final TSH
Conclusion: febuxostat increases TSH levels, both baseline TSH and febuxostat dose being associated with the increase. No patient with TSH above UNLs showed altered T4 levels or symptoms of thyroid dysfunction.
Disclosure:
F. Perez-Ruiz,
Menarini,
5,
Ardea Biosciences,
5,
Novartis Pharmaceutical Corporation,
5,
Savient,
5,
Menarini,
8,
Ardea Biosciences,
8,
Savient,
8,
Novartis Pharmaceutical Corporation,
8;
A. M. Herrero-Beites,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increase-of-thyroid-stimulating-hormone-in-patients-on-febuxostat-treatment/