Session Information
Session Type: ACR Late-breaking Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase (JAK) inhibitor that preferentially inhibits signaling by JAK3 and JAK1, with functional selectivity over JAK2. Potential increased risk of venous thromboembolic events (VTE) in patients (pts) with rheumatoid arthritis (RA) has been reported for a JAK 1/2 inhibitor.1 To assess VTE risk with tofacitinib, data were reviewed from the tofacitinib development program in RA, psoriasis (PsO), psoriatic arthritis (PsA), and ulcerative colitis (UC).
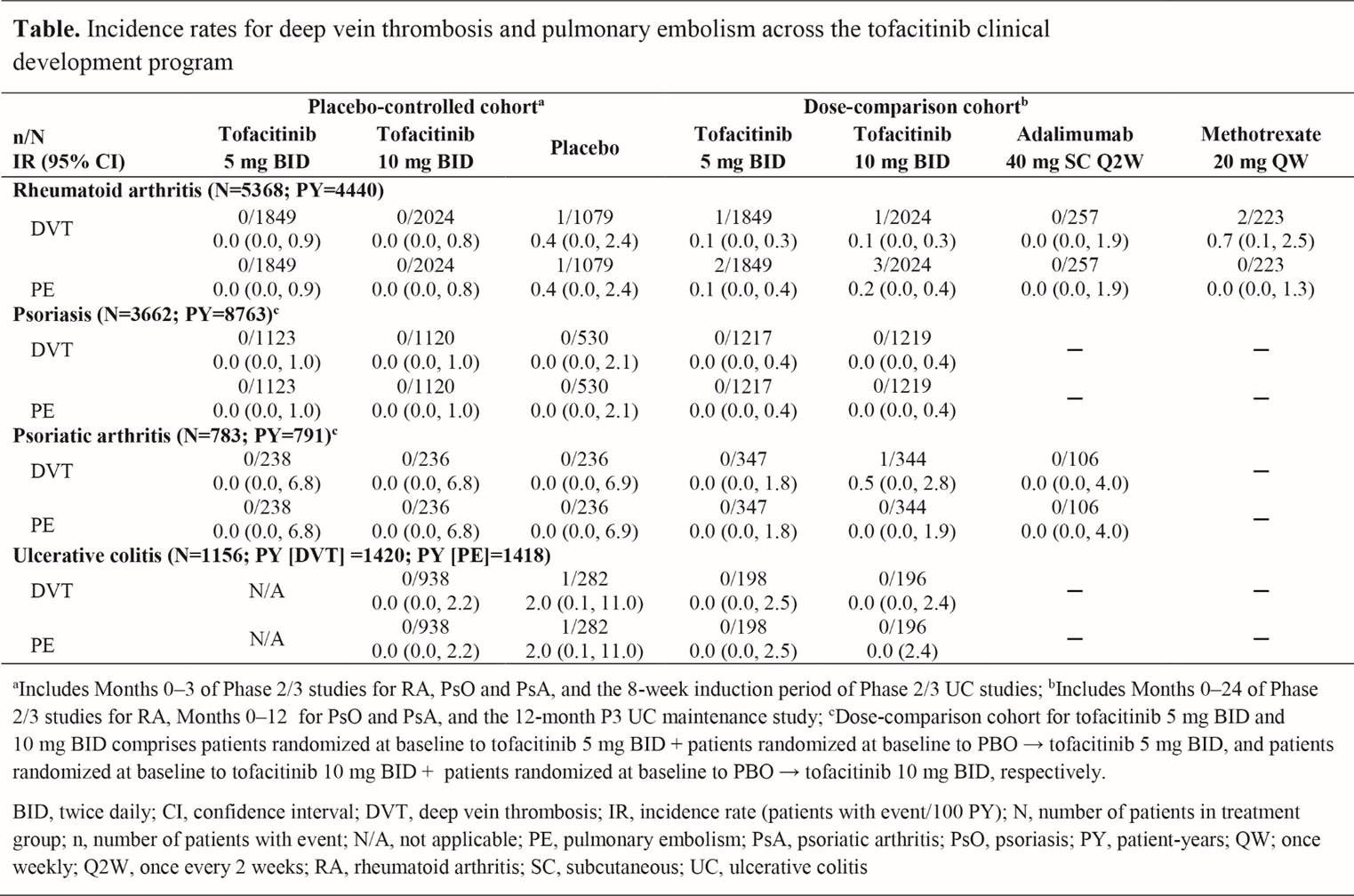
Methods: Data from Phase (P) 2 (RA, PsO, UC) and P3 (RA, PsO, PsA, UC) randomized clinical studies of tofacitinib as monotherapy or in combination with conventional synthetic (cs)DMARDs were included. Two cohorts were defined; 1) the placebo (PBO)-controlled cohort: pts randomized to tofacitinib 5 or 10 mg BID, or PBO up to Month (M) 3 in RA, PsO, and PsA studies, and pts randomized to tofacitinib 10 mg BID or PBO for the 9-week induction period in UC studies; 2) the dose-comparison cohort: pts randomized to tofacitinib 5 or 10 mg BID, adalimumab (ADA) 40 mg SC Q2W (RA and PSA only) or methotrexate (MTX) 20 mg QW (RA only) throughout the P2/3 studies for RA (up to M24), PsO (up to M12), and PsA (up to M12), and for the 12-month P3 UC maintenance study. First deep vein thrombosis (DVT) and pulmonary embolism (PE) events were identified using the MedDRA embolic and thrombotic SMQ preferred terms restricted to the respiratory, thoracic, mediastinal, and vascular disorder System Organ Classes; incidence rates (IRs; pts with events/100 pt-years) were based on single events occurring during treatment or ≤28 days after the last dose or up to the cohort cut-off date. IRs for PE in pts with RA were compared with Corrona Registry data.
Results: Up to M3 in the PBO-controlled cohort, DVT and PE were both independently reported in 1 pt with RA and 1 with UC, who both received PBO; no pts receiving tofacitinib had DVT or PE events (Table). In the dose-comparison cohort there were 2 DVT events in tofacitinib-treated pts with RA (5 mg BID, n=1; 10 mg BID n=1) and 1 DVT event in a pt with PsA (tofacitinib 10 mg BID) (Table). IRs were 0.1 (95% CI: 0.0, 0.3) for both tofacitinib doses in RA, and 0.5 (95% CI: 0.0, 2.8) for tofacitinib 10 mg BID in PsA. Five PE events occurred in the dose-comparison cohort, all in RA (5 mg BID, n=2; 10 mg BID, n=3). IRs were 0.1 (95% CI: 0.0, 0.4) for tofacitinib 5 mg BID and 0.2 (95% CI: 0.0, 0.4) for 10 mg BID. IRs for PE with tofacitinib in RA were similar to those reported by the Corrona Registry in pts with RA treated with tofacitinib (0.1 [95% CI: 0.0, 0.4]), biologic DMARDs (0.2 [95% CI: 0.1, 0.3]), and csDMARDs (0.2 [95% CI: 0.0, 0.5]). DVT were reported twice with MTX, and none with ADA.
Conclusion: Analysis of DVT and PE across randomized clinical studies for RA, PsO, PsA, and UC showed no evidence of an increased risk of events with tofacitinib.
1. Kremer K, et al. EULAR 2017 Abstract, FRI0090.
Disclosure: P. J. Mease, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, Sun Pharmaceutical, UCB, 2,AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, Sun Pharmaceutical, UCB, 5,AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Novartis, Pfizer Inc, UCB, 8; J. Kremer, Corrona, LLC, 1,Corrona, LLC, 3,AbbVie, Amgen, BMS, Genentech, Lilly, Regeneron, Sanofi, Pfizer, 5,AbbVie, Genentech, Lilly, Novartis, Pfizer, 2; S. Cohen, AbbVie, Amgen, Boehringer Ingelheim, Gilead, Merck, Pfizer Inc, 5,AbbVie, Amgen, Boehringer Ingelheim, Gilead, Merck, Pfizer Inc, 9; J. R. Curtis, Amgen, 2,Corrona, 2,Crescendo Bio, 2,Pfizer Inc, 2,AbbVie, 5,Amgen, 5,Bristol-Myers Squibb, 5,Corrona, 5,Eli Lilly and Company, 5,Janssen Pharmaceutica Product, L.P., 5,Myriad, 5,Pfizer Inc, 5,Roche Pharmaceuticals, 5,UCB, 5; C. Charles-Schoeman, AbbVie, Bristol-Myers Squibb, Pfizer Inc, 2,Amgen, Pfizer Inc, Regeneron-Sanofi, 3; E. V. Loftus, AbbVie, 5,Amgen, 5,CVS Caremark, 5,Eli Lilly and Company, 5,Janssen Pharmaceutica Product, L.P., 5,Mesoblast, 5,Pfizer Inc, 5,Salix, 5,Takeda, 5,UCB, 5,AbbVie, 2,Amgen, 2,Celgene, 2,Genentech and Biogen IDEC Inc., 2,Gilead, 2,Janssen Pharmaceutica Product, L.P., 2,MedImmune, 2,Pfizer Inc, 2,Receptos, 2,Robarts Clinical Trials, 2,Seres, 2,Takeda, 2,UCB, 2; J. D. Greenberg, Corrona, LLC, 1,Corrona, LLC, 3,Eli Lilly, Genentech, Janssen, Novartis, Pfizer, 5; N. Palmetto, Pfizer Inc, 1,Pfizer Inc, 3; K. S. Kanik, Pfizer Inc, 1,Pfizer Inc, 3; D. Graham, Pfizer Inc, 1,Pfizer Inc, 3; C. Wang, Pfizer Inc, 1,Pfizer Inc, 3; P. Biswas, Pfizer Inc, 1,Pfizer Inc, 3; G. Chan, Pfizer Inc, 1,Pfizer Inc, 3; R. DeMasi, Pfizer Inc, 1,Pfizer Inc, 3; H. Valdez, Pfizer Inc, 1,Pfizer Inc, 3; T. Hendrikx, Pfizer Inc, 1,Pfizer Inc, 3; T. V. Jones, Pfizer Inc, 1,Pfizer Inc, 3.
To cite this abstract in AMA style:
Mease PJ, Kremer J, Cohen S, Curtis JR, Charles-Schoeman C, Loftus EV, Greenberg JD, Palmetto N, Kanik KS, Graham D, Wang C, Biswas P, Chan G, DeMasi R, Valdez H, Hendrikx T, Jones TV. Incidence of Thromboembolic Events in the Tofacitinib Rheumatoid Arthritis, Psoriasis, Psoriatic Arthritis and Ulcerative Colitis Development Programs [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/incidence-of-thromboembolic-events-in-the-tofacitinib-rheumatoid-arthritis-psoriasis-psoriatic-arthritis-and-ulcerative-colitis-development-programs/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-thromboembolic-events-in-the-tofacitinib-rheumatoid-arthritis-psoriasis-psoriatic-arthritis-and-ulcerative-colitis-development-programs/