Session Information
Date: Monday, October 22, 2018
Title: 4M089 ACR Abstract: Spondyloarthritis Incl PsA–Clinical III: Treatment of Axial SpA (1864–1869)
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Increased incidence of IBD, such as Crohn’s disease (CD) and ulcerative colitis (UC), has been observed in patients (pts) with PsA or plaque psoriasis (PsO) compared to those without. A greater incidence of IBD has been reported in pts with coexistent PsA and PsO.1 Animal and human studies suggest a role for IL-17 in PsA, PsO, and IBD.2 We report the incidence of IBD in pts treated with ixekizumab (IXE), a high-affinity mAb selectively targeting IL-17A,3 in pts with PsA, PsO, or comorbid PsA and PsO.
Methods: Adverse events of suspected IBD were collected for IXE-treated pts in an integrated database of 3 (2 randomized, controlled) PsA phase 3 studies (SPIRIT-P1, -P2, -P3) and 12 studies in moderate-to-severe plaque PsO (1-phase 1, 1-phase 2, and 10-phase 3 [includes UNCOVER-1, -2, -3, -A, and -J]). Suspected IBD cases were adjudicated by internationally recognized classification criteria (EPIMAD). Adjudication data were summarized by indication. Percentage and exposure-adjusted incidence rates (IR) per 1000 patient year (PY) were calculated.
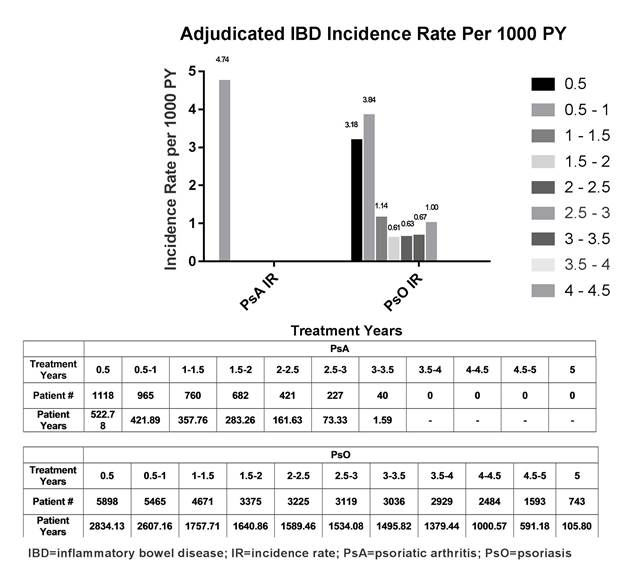
Results: During the double-blind (DB) placebo controlled period of PsO studies (Weeks 0-12), 3 (0.1%; IR=5.6/1000PY) IXE-treated pts had IBD events compared to none in placebo or etanercept arms ; none were detected in any arm of the PsA DB study periods (Weeks 0-24). Of 1118 pts in PsA trials (1822 PY) who received at least one dose of IXE, 2 pts (IR=1.1/1000PY;1 CD, 1 UC) had adjudicated IBD; both events occurred at 6 months to 1 year of treatment with IXE 80 mg every 2 weeks. Of 5898 pts in PsO trials (16313 PY) who received at least one dose of IXE, 26 pts (IR=1.6/1000PY; 7 CD, 19 UC) had adjudicated IBD. In 19 of 26 pts, events occurred within 1 year of IXE exposure (9 at <6 months; 10 at 6 months to 1 year). Among 28 adjudicated pts, 19 (PsO) were previously adjudicated; 4 and 9 (7 PsO; 2 PsA) were newly adjudicated for IBD.
Conclusion: Events of CD and UC occurred infrequently among IXE-treated pts from 3 PsA trials and 12 PsO trials. Additional analysis and adjudication of longer-term data is currently ongoing and are required to further understand the relationship between IBD and IXE treatment.
References:
1. Egeberg A, et al. Br J Dermatol 2016;175(3):487-92.
2. Martin DA, et al. J Invest Dermatol 2013;133(1):17-26.
3. Gordon KB, et al. N Engl J Med 2016;375(4):345-56.
4. Reich K, et al. J Am Acad Dermatol 2017;76(3):441-48 e2.
Figure.
To cite this abstract in AMA style:
Genovese MC, Colombel JF, Gellett AM, Xu W, Hardin D. Incidence of Inflammatory Bowel Disease Among Patients Treated with Ixekizumab: An Update on Adjudicated Data from an Integrated Database of Patients with Psoriasis and Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/incidence-of-inflammatory-bowel-disease-among-patients-treated-with-ixekizumab-an-update-on-adjudicated-data-from-an-integrated-database-of-patients-with-psoriasis-and-psoriatic-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-inflammatory-bowel-disease-among-patients-treated-with-ixekizumab-an-update-on-adjudicated-data-from-an-integrated-database-of-patients-with-psoriasis-and-psoriatic-arthritis/